Neural Network Reorganization Analysis During an Auditory Oddball Task in Schizophrenia Using Wavelet Entropy
Abstract
:1. Introduction
2. Materials
2.1. Subjects
| Characteristic | SCH Patients | Controls |
|---|---|---|
| Age (years)* | 35.45 ± 12.07 | 33.35 ± 12.26 |
| Gender (Male:Female) | 14:6 | 14:6 |
| PANSS-Positive | 18.87 ± 4.39 | NA |
| PANSS-Negative | 20.93 ± 5.76 | NA |
| PANSS-Total | 74.47 ± 17.70 | NA |
2.2. Recording and Preprocessing of ERP Signals
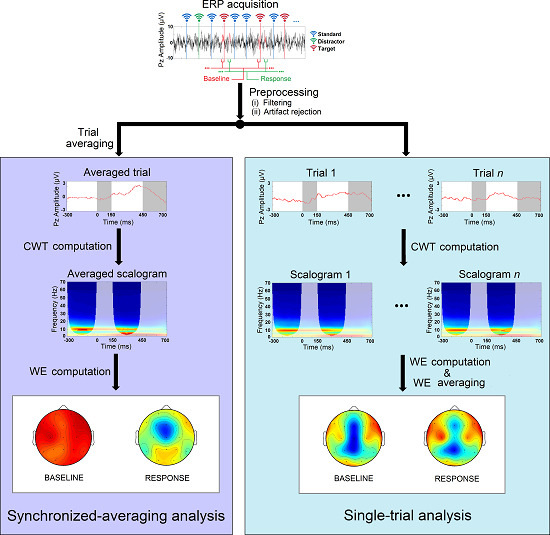
3. Methods

3.1. Continuous Wavelet Transform
3.2. Wavelet Entropy
3.3. Statistical Analysis
4. Results
4.1. Single-Trial ERP Analysis


4.2. Synchronized-Averaging ERP Analysis

5. Discussion

6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM-5; American Psychiatric Publishing: Arlington, VA, USA, 2013; p. 991. [Google Scholar]
- Marshall, M.; Lewis, S.; Lockwood, A.; Drake, R.; Jones, P.; Croudace, T. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: A systematic review. Arch. Gen. Psychiatry 2005, 62, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chant, D.; Welham, J.; McGrath, J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005, 2, 0413–0433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. The disconnection hypothesis. Schizophr. Res. 1998, 30, 115–125. [Google Scholar] [CrossRef]
- Bogerts, B.; Ashtari, M.; Degreef, G.; Alvir, J.; Bilder, R.M.; Lieberman, J.A. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. Neuroimaging 1990, 35, 1–13. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. Neural Synchrony in Brain Disorders: Relevance for Cognitive Dysfunctions and Pathophysiology. Neuron 2006, 52, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, M.P.; Mandl, R.C.W.; Stam, C.J.; Kahn, R.S.; Hulshoff Pol, H.E. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J. Neurosci. 2010, 30, 15915–15926. [Google Scholar] [CrossRef] [PubMed]
- Molina, V.; Hernández, J.A.; Sanz, J.; Paniagua, J.C.; Hernández, A.I.; Martín, C.; Matías, J.; Calama, J.; Bote, B. Subcortical and cortical gray matter differences between Kraepelinian and non-Kraepelinian schizophrenia patients identified using voxel-based morphometry. Psychiatry Res. Neuroimaging 2010, 184, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Gur, R.E.; Gur, R.C. Functional magnetic resonance imaging in schizophrenia. Dialogues Clin. Neurosci. 2010, 12, 333–343. [Google Scholar] [PubMed]
- Kubicki, M.; McCarley, R.; Westin, C.-F.; Park, H.-J.; Maier, S.; Kikinis, R.; Jolesz, F.A.; Shenton, M.E. A review of diffusion tensor imaging studies in schizophrenia. J. Psychiatr. Res. 2007, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010, 11, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Mathalon, D.H.; Ford, J.M.; Pfefferbaum, A. Trait and state aspects of P300 amplitude reduction in schizophrenia: A retrospective longitudinal study. Biol. Psychiatry 2000, 47, 434–449. [Google Scholar] [CrossRef]
- O’Donnell, B.F.; Faux, S.F.; McCarley, R.W.; Kimble, M.O.; Salisbury, D.F.; Nestor, P.G.; Kikinis, R.; Jolesz, F.A.; Shenton, M.E. Increased rate of P300 latency prolongation with age in schizophrenia. Electrophysiological evidence for a neurodegenerative process. Arch. Gen. Psychiatry 1995, 52, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Baldeweg, T.; Klugman, A.; Gruzelier, J.H.; Hirsch, S.R. Impairment in frontal but not temporal components of mismatch negativity in schizophrenia. Int. J. Psychophysiol. 2002, 43, 111–122. [Google Scholar] [CrossRef]
- Kircher, T.T.J.; Rapp, A.; Grodd, W.; Buchkremer, G.; Weiskopf, N.; Lutzenberger, W.; Ackermann, H.; Mathiak, K. Mismatch Negativity Responses in Schizophrenia: A Combined fMRI and Whole-Head MEG Study. Am. J. Psychiatry 2004, 161, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. Disconnection and cognitive dysmetria in schizophrenia. Am. J. Psychiatry 2005, 162, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Haenschel, C.; Nikolić, D.; Singer, W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 2008, 34, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, A.; Picton, T.W. EEG spectral dynamics during discrimination of auditory and visual targets. Cogn. Brain Res. 2005, 24, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Roux, F.; Rodriguez, E.; Rotarska-Jagiela, A.; Singer, W. Neural synchrony and the development of cortical networks. Trends Cogn. Sci. 2010, 14, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr. Opin. Neurobiol. 2013, 23, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Cho, R.Y.; Mizuno, T.; Kikuchi, M.; Murata, T.; Takahashi, K.; Wada, Y. Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: A multiscale entropy analysis. Neuroimage 2010, 51, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, M.; Boostani, R.; Sabeti, M.; Taghavi, S.M.A. Usefulness of approximate entropy in the diagnosis of schizophrenia. Iran. J. Psychiatry Behav. Sci. 2011, 5, 62–70. [Google Scholar] [PubMed]
- Bassett, D.S.; Nelson, B.G.; Mueller, B.A.; Camchong, J.; Lim, K.O. Altered resting state complexity in schizophrenia. Neuroimage 2012, 59, 2196–2207. [Google Scholar] [CrossRef] [PubMed]
- Bachiller, A.; Lubeiro, A.; Díez, A.; Suazo, V.; Domínguez, C.; Blanco, J.A.; Ayuso, M.; Hornero, R.; Poza, J.; Molina, V. Decreased entropy modulation of EEG response to novelty and relevance in schizophrenia during a P300 task. Eur. Arch. Psychiatry Clin. Neurosci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bachiller, A.; Díez, A.; Suazo, V.; Domínguez, C.; Ayuso, M.; Hornero, R.; Poza, J.; Molina, V. Decreased spectral entropy modulation in patients with schizophrenia during a P300 task. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Le Van Quyen, M.; Foucher, J.; Lachaux, J.; Rodriguez, E.; Lutz, A.; Martinerie, J.; Varela, F.J. Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. J. Neurosci. Methods 2001, 111, 83–98. [Google Scholar] [CrossRef]
- Mallat, S. A Wavelet Tour of Signal Processing; Academic Press: Waltham, MA, USA, 1999; pp. 20–41. [Google Scholar]
- Bachiller, A.; Poza, J.; Gómez, C.; Molina, V.; Suazo, V.; Hornero, R. A comparative study of event-related coupling patterns during an auditory oddball task in schizophrenia. J. Neural Eng. 2015, 12, 016007. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Quiroga, R.Q.; Rosso, O.A.; Başar, E.; Schürmann, M. Wavelet entropy in event-related potentials: A new method shows ordering of EEG oscillations. Biol. Cybern. 2001, 84, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ergen, M.; Marbach, S.; Brand, A.; Başar-Eroǧlu, C.; Demiralp, T. P3 and delta band responses in visual oddball paradigm in schizophrenia. Neurosci. Lett. 2008, 440, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Opler, L.A.; Lindenmayer, J.P. The Positive and Negative Syndrome Scale (PANSS): Rationale and standardisation. Br. J. Psychiatry 1989, 155, 59–65. [Google Scholar]
- Bledowski, C.; Prvulovic, D.; Hoechstetter, K.; Scherg, M.; Wibral, M.; Goebel, R.; Linden, D.E. Localizing P300 generators in visual target and distractor processing: A combined event-related potential and functional magnetic resonance imaging study. J. Neurosci. 2004, 24, 9353–9360. [Google Scholar] [CrossRef] [PubMed]
- Keren, A.S.; Yuval-Greenberg, S.; Deouell, L.Y. Saccadic spike potentials in gamma-band EEG: Characterization, detection and suppression. Neuroimage 2010, 49, 2248–2263. [Google Scholar] [CrossRef] [PubMed]
- Roach, B.J.; Mathalon, D.H. Event-related EEG time-frequency analysis: An overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr. Bull. 2008, 34, 907–926. [Google Scholar] [CrossRef] [PubMed]
- Makeig, S.; Debener, S.; Onton, J.; Delorme, A. Mining event-related brain dynamics. Trends Cogn. Sci. 2004, 8, 204–210. [Google Scholar] [CrossRef] [PubMed]
- David, O.; Harrison, L.; Friston, K.J. Modelling event-related responses in the brain. Neuroimage 2005, 25, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.; Garcia, H.; Quiroga, R.Q.; Romanelli, L.; Rosso, O.A. Stationarity of the EEG series. IEEE Eng. Med. Biol. Mag. 1995, 14, 395–399. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G. A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Rosso, O.A.; Blanco, S.; Yordanova, J.; Kolev, V.; Figliola, A.; Schürmann, M.; Başar, E. Wavelet entropy: A new tool for analysis of short duration brain electrical signals. J. Neurosci. Methods 2001, 105, 65–75. [Google Scholar] [CrossRef]
- Ford, J.M.; Roach, B.J.; Faustman, W.O.; Mathalon, D.H. Synch before you speak: Auditory hallucinations in schizophrenia. Am. J. Psychiatry 2007, 164, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Lage-Castellanos, A.; Martínez-Montes, E.; Hernández-Cabrera, J.A.; Galán, L. False discovery rate and permutation test: An evaluation in ERP data analysis. Stat. Med. 2010, 29, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ferrarelli, F.; Massimini, M.; Peterson, M.J.; Riedner, B.A.; Lazar, M.; Murphy, M.J.; Huber, R.; Rosanova, M.; Alexander, A.L.; Kalin, N.; Tononi, G. Reduced Evoked Gamma Oscillations in the Frontal Cortex in Schizophrenia Patients a TMS EEG Study. Am. J. Psychiatry 2008, 165, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Tallon-Baudry, C.; Bertrand, O.; Delpuech, C.; Pernier, J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J. Neurosci. 1996, 16, 4240–4249. [Google Scholar] [PubMed]
- Stefanics, G.; Hangya, B.; Hernádi, I.; Winkler, I.; Lakatos, P.; Ulbert, I. Phase entrainment of human delta oscillations can mediate the effects of expectation on reaction speed. J. Neurosci. 2010, 30, 13578–13585. [Google Scholar] [CrossRef] [PubMed]
- Bramon, E.; Rabe-Hesketh, S.; Sham, P.; Murray, R.M.; Frangou, S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr. Res. 2004, 70, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Von Stein, A.; Chiang, C.; König, P. Top-down processing mediated by interareal synchronization. Proc. Natl. Acad. Sci. USA 2000, 97, 14748–14753. [Google Scholar] [CrossRef] [PubMed]
- Tallon-Baudry, C.; Bertrand, O. Oscillatory gamma activity and its role in object representation. Trends Cogn. Sci. 1999, 3, 151–162. [Google Scholar] [CrossRef]
- Potts, G.F.; Hirayasu, Y.; O’Donnell, B.F.; Shenton, M.E.; McCarley, R.W. High-density recording and topographic analysis of the auditory oddball event-related potential in patients with schizophrenia. Biol. Psychiatry 1998, 44, 982–989. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Palaniyappan, L.; Doege, K.; Mallikarjun, P.; Liddle, E. Cortical thickness and oscillatory phase resetting: A proposed mechanism of salience network dysfunction in schizophrenia. Psychiatriki 2012, 23, 117–129. [Google Scholar] [PubMed]
- White, T.P.; Joseph, V.; Francis, S.T.; Liddle, P.F. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr. Res. 2010, 123, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Mormann, F.; Fell, J.; Axmacher, N.; Weber, B.; Lehnertz, K.; Elger, C.E.; Fernández, G. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus 2005, 15, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ramirez, M.; Kelly, S.P.; Molholm, S.; Sehatpour, P.; Schwartz, T.H.; Foxe, J.J. Oscillatory Sensory Selection Mechanisms during Intersensory Attention to Rhythmic Auditory and Visual Inputs: A Human Electrocorticographic Investigation. J. Neurosci. 2011, 31, 18556–18567. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Pilar, J.; Poza, J.; Bachiller, A.; Gómez, C.; Molina, V.; Hornero, R. Neural Network Reorganization Analysis During an Auditory Oddball Task in Schizophrenia Using Wavelet Entropy. Entropy 2015, 17, 5241-5256. https://0-doi-org.brum.beds.ac.uk/10.3390/e17085241
Gomez-Pilar J, Poza J, Bachiller A, Gómez C, Molina V, Hornero R. Neural Network Reorganization Analysis During an Auditory Oddball Task in Schizophrenia Using Wavelet Entropy. Entropy. 2015; 17(8):5241-5256. https://0-doi-org.brum.beds.ac.uk/10.3390/e17085241
Chicago/Turabian StyleGomez-Pilar, Javier, Jesús Poza, Alejandro Bachiller, Carlos Gómez, Vicente Molina, and Roberto Hornero. 2015. "Neural Network Reorganization Analysis During an Auditory Oddball Task in Schizophrenia Using Wavelet Entropy" Entropy 17, no. 8: 5241-5256. https://0-doi-org.brum.beds.ac.uk/10.3390/e17085241