Effect of a Percutaneous Coronary Intervention Procedure on Heart Rate Variability and Pulse Transit Time Variability: A Comparison Study Based on Fuzzy Measure Entropy
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Protocol
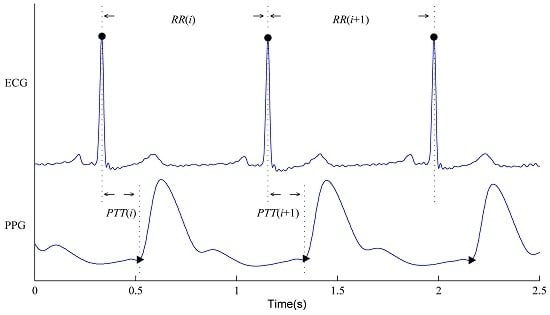
2.3. RR and PTT Series Construction
2.4. Variability Indices for HRV and PTTV
2.5. Statistical Analysis
3. Results
3.1. RR and PTT Intervals: Comparison before and after PCI Procedure
3.2. HRV and PTTV Results: Comparison between before and after PCI Procedure
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
Appendix A1. SDTS
Appendix A2. FuzzyMEn
References
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Mortara, A.; Capomolla, S.; Febo, O.; Ferrari, R.; Franchini, M.; Gnemmi, M.; Opasich, C.; et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003, 107, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zhang, C.Q.; Zhang, L.; Zhao, L.N.; Liu, C.C.; Wang, H.J. Measuring synchronization in coupled simulation and coupled cardiovascular time series: A comparison of different cross entropy measures. Biomed. Signal Process. Control 2015, 21, 49–57. [Google Scholar] [CrossRef]
- Janowska-Kulinska, A.; Torzynska, K.; Markiewicz-Grochowalska, A.; Sowinska, A.; Majewski, M.; Jerzykowska, O.; Pawlak-Bus, K.; Kramer, L.; Moczko, J.; Siminiak, T. Changes in heart rate variability caused by coronary angioplasty depend on the localisation of coronary lesions. Kardiologia Polska 2009, 67, 130–138. [Google Scholar] [PubMed]
- Liu, C.Y.; Liu, C.C.; Li, L.P.; Zhang, Q.G.; Li, B. Systolic and Diastolic Time Interval Variability Analysis and Their Relations with Heart Rate Variability. In Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 1–4.
- Bigger, J.T.; Fleiss, J.L.; Rolnitzky, L.M.; Steinman, R.C. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation 1993, 88, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, C.Y.; Li, K.; Zheng, D.C.; Liu, C.C.; Hou, Y. Assessing the complexity of short-term heartbeat interval series by distribution entropy. Med. Biol. Eng. Comput. 2015, 53, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zhao, L.N. Using Fuzzy Measure Entropy to Improve the Stability of Traditional Entropy Measures. In Proceedings of the Computing in Cardiology, Hangzhou, China, 18–21 September 2011; pp. 681–684.
- Chen, W.T.; Zhuang, J.; Yu, W.X.; Wang, Z.Z. Measuring complexity using fuzzyen, apen, and sampen. Med. Eng. Phys. 2009, 31, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kanadasi, M.; Kudaiberdieva, G.; Birand, A. Effect of the final coronary arterial diameter after coronary angioplasty on heart rate variability responses. Ann. Noninvasive Electrocardiol. 2002, 7, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Lakusic, N.; Mahovic, D.; Kruzliak, P.; Habek, J.C.; Novak, M.; Cerovec, D. Changes in heart rate variability after coronary artery bypass grafting and clinical importance of these findings. BioMed Res. Int. 2015, 2015, 680515. [Google Scholar] [CrossRef] [PubMed]
- Laitio, T.T.; Huikuri, H.V.; Kentala, E.S.; Makikallio, T.H.; Jalonen, J.R.; Helenius, H.; Sariola-Heinonen, K.; Yli-Mayry, S.; Scheinin, H. Correlation properties and complexity of perioperative RR-interval dynamics in coronary artery bypass surgery patients. Anesthesiology 2000, 93, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Hogue, C.W.J.; Stein, P.K.; Apostolidou, I.; Lappas, D.G.; Kleiger, R.E. Alterations in temporal patterns of heart rate variability after coronary artery bypass graft surgery. Anesthesiology 1994, 81, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.K.; Vikman, S.; Laurikka, J.; Pehkonen, E.; Iivainen, T.; Huikuri, H.V.; Tarkka, M.R. Nonlinear heart rate variability in cabg patients and the preconditioning effect. Eur. J. Cardiothorac. Surg. 2005, 28, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Lakusic, N.; Slivnjak, V.; Baborski, F.; Sonicki, Z. Heart rate variability in patients after cardiac valve surgery. Cent. Eur. J. Med. 2008, 3, 65–70. [Google Scholar] [CrossRef]
- Tseng, C.D.; Wang, T.L.; Lin, J.L.; Hsu, K.L.; Chiang, F.T.; Tseng, Y.Z. The cause-effect relationship of sympathovagal activity and the outcome of percutaneous transluminal coronary angioplasty. Jpn. Heart J. 1996, 37, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zheng, D.C.; Zhao, L.N.; Li, P.; Li, B.; Murray, A.; Liu, C.C. Elastic properties of peripheral arteries in heart failure patients in comparison with normal subjects. J. Physiol. Sci. 2013, 63, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Gharacholou, S.M.; Scott, C.G.; Borlaug, B.A.; Kane, G.C.; McCully, R.B.; Oh, J.K.; Pellikka, P.A. Relationship between diastolic function and heart rate recovery after symptom-limited exercise. J. Card. Fail. 2012, 18, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Roesch, N.; Harpes, P.; Körtke, H.; Plumer, P.; Saberin, A.; Chakoutio, V.; Oundjede, D.; Delagardelle, C.; Beissel, J.; et al. Relationship between pulse transit time and blood pressure is impaired in patients with chronic heart failure. Clin. Res. Cardiol. 2010, 99, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M.; Goldberger, A.L. Physiological time-series analysis: What does regularity quantify? Am. J. Physiol. Heart Circ. Physiol. 1994, 266, H1643–H1656. [Google Scholar]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [PubMed]
- Liu, C.Y.; Liu, C.C.; Shao, P.; Li, L.P.; Sun, X.; Wang, X.P.; Liu, F. Comparison of different threshold values r for approximate entropy: Application to investigate the heart rate variability between heart failure and healthy control groups. Physiol. Meas. 2011, 32, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, X.; Kanters, J.K.; Solomon, I.C.; Chon, K.H. Automatic selection of the threshold value R for approximate entropy. IEEE Trans. Biomed. Eng. 2008, 55, 1966–1972. [Google Scholar] [PubMed]
- Zhao, L.N.; Wei, S.S.; Zhang, C.Q.; Zhang, Y.T.; Jiang, X.E.; Liu, F.; Liu, C.Y. Determination of sample entropy and fuzzy measure entropy parameters for distinguishing congestive heart failure from normal sinus rhythm subjects. Entropy 2015, 17, 6270–6288. [Google Scholar] [CrossRef]
- Liu, C.Y.; Li, K.; Zhao, L.N.; Liu, F.; Zheng, D.C.; Liu, C.C.; Liu, S.T. Analysis of heart rate variability using fuzzy measure entropy. Comput. Biol. Med. 2013, 43, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Wang, Z.Z.; Xie, H.B.; Yu, W.X. Characterization of surface emg signal based on fuzzy entropy. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, C.Y.; Wang, X.P.; Zheng, D.C.; Li, Y.Y.; Liu, C.C. A low-complexity data-adaptive approach for premature ventricular contraction recognition. Signal Image Video Process. 2014, 8, 111–120. [Google Scholar] [CrossRef]
- Liu, C.Y.; Li, L.P.; Zhao, L.N.; Zheng, D.C.; Li, P.; Liu, C.C. A combination method of improved impulse rejection filter and template matching for identification of anomalous intervals in electrocardiographic RR sequences. J. Med. Biol. Eng. 2012, 32, 245–250. [Google Scholar] [CrossRef]
- Yambe, T.; Shiraishi, Y.; Saijo, Y.; Liu, H.; Nitta, S.; Imachi, K.; Baba, A.; Yamaguchi, T.; Sugawara, S.; Katahira, Y.; et al. Clinical research on the accuracy in determining the pulse wave rising point. SCR Med. 2009, 82, 164–174. [Google Scholar]
- Shu, A.; Zhan, L.; Fang, H.; Lv, E.; Chen, X.; Zhang, M.; Wang, Q. Evaluation of remifentanil anesthesia for off-pump coronary artery bypass grafting surgery using heart rate variability. Exp. Ther. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.P.S.; Moreno, A.M.; Cravo, S.L.D.; Nobrega, A.C.L. Coronary artery bypass surgery and longitudinal evaluation of the autonomic cardiovascular function. Crit. Care 2005, 9, R124–R131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharwood-Smith, G.; Bruce, J.; Drummond, G. Assessment of pulse transit time to indicate cardiovascular changes during obstetric spinal anaesthesia. Br. J. Anaesth. 2006, 96, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zheng, Y.Y.; Ma, Z.C.; Sun, Y.N. Radial pulse transit time is an index of arterial stiffness. Hypertens. Res. 2011, 34, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zheng, D.C.; Zhao, L.N.; Liu, C.C. Gaussian fitting for carotid and radial artery pressure waveforms: Comparison between normal subjects and heart failure patients. Biomed. Mater. Eng. 2014, 24, 271–277. [Google Scholar] [PubMed]
- Liu, C.Y.; Zheng, D.C.; Murray, A. Arteries stiffen with age, but can retain an ability to become more elastic with applied external cuff pressure. Medicine 2015, 94, e1831. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [PubMed]
- Aktaruzzaman, M.; Sassi, R. Parametric estimation of sample entropy in heart rate variability analysis. Biomed. Signal Process. Control 2014, 14, 141–147. [Google Scholar] [CrossRef]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Tocci, G.; Barbato, E.; Coluccia, R.; Modestino, A.; Pagliaro, B.; Mastromarino, V.; Giovannelli, F.; Berni, A.; Volpe, M. Blood pressure levels at the time of percutaneous coronary revascularization and risk of coronary in-stent restenosis. Am. J. Hypertens. 2015, 29, 509–518. [Google Scholar] [CrossRef] [PubMed]




| Variables | Value | Range |
|---|---|---|
| Number (M/F) | 16 (13/3) | - |
| Age (year) | 61 ± 9 | 44–80 |
| Height (cm) | 169 ± 7 | 157–181 |
| Weight (kg) | 72 ± 9 | 56–90 |
| BMI (kg/m2) | 25.1 ± 2.5 | 20.8–28.7 |
| SBP (mmHg) | 130 ± 14 | 111–150 |
| DBP (mmHg) | 79 ± 10 | 67–103 |
| LVEF (%) | 61 ± 6 | 50–72 |
| Subject No. | Time Interval between the Singal Recording and PCI Intervention Operation | |
|---|---|---|
| Before PCI (h) | After PCI (h) | |
| 1 | 19.6 | 18.8 |
| 2 | 23.6 | 24.0 |
| 3 | 21.6 | 22.1 |
| 4 | 21.7 | 22.5 |
| 5 | 19.7 | 20.3 |
| 6 | 21.6 | 19.4 |
| 7 | 17.3 | 18.5 |
| 8 | 24.0 | 23.3 |
| 9 | 18.3 | 21.8 |
| 10 | 23.2 | 19.4 |
| 11 | 23.7 | 24.0 |
| 12 | 16.0 | 21.6 |
| 13 | 18.0 | 18.9 |
| 14 | 18.5 | 8.9 |
| 15 | 19.4 | 22.7 |
| 16 | 23.3 | 20.6 |
| Average | 20.6 | 20.4 |
| SD | 2.6 | 3.6 |
| Parameters | Before PCI | After PCI | p-Value |
|---|---|---|---|
| RR (ms) | 973 ± 85 | 907 ± 100 | <0.05 |
| PTT (ms) | 207 ± 18 | 214 ± 19 | <0.01 |
| SBP (mmHg) | 130 ± 14 | 131 ± 18 | 0.4 |
| DBP (mmHg) | 79 ± 10 | 79 ± 11 | 0.9 |
| Variables | HRV | PTTV | ||||
|---|---|---|---|---|---|---|
| Before PCI | After PCI | p-Value | Before PCI | After PCI | p-Value | |
| n = 200 | ||||||
| SDTS (ms) | 23.8 ± 10.4 | 19.2 ± 7.4 | 0.10 | 4.29 ± 1.77 | 4.29 ± 1.83 | 0.99 |
| FuzzyMEn | 0.83 ± 0.24 | 0.64 ± 0.34 | <0.05 | 1.09 ± 0.13 | 0.96 ± 0.16 | <0.01 |
| n = 100 | ||||||
| SDTS (ms) | 23.3 ± 10.1 | 18.1 ± 6.8 | <0.05 | 4.16 ± 1.68 | 4.20 ± 1.91 | 0.90 |
| FuzzyMEn | 0.85 ± 0.27 | 0.70 ± 0.30 | <0.05 | 1.11 ± 0.12 | 0.99 ± 0.14 | <0.01 |
| n = 50 | ||||||
| SDTS (ms) | 21.7 ± 10.2 | 16.7 ± 6.2 | 0.06 | 4.06 ± 1.66 | 4.09 ± 1.88 | 0.93 |
| FuzzyMEn | 0.95 ± 0.23 | 0.82 ± 0.27 | <0.05 | 1.15 ± 0.13 | 1.04 ± 0.15 | <0.01 |
| n = 25 | ||||||
| SDTS (ms) | 20.8 ± 10.8 | 15.3 ± 5.7 | <0.05 | 3.99 ± 1.66 | 3.97 ± 1.86 | 0.95 |
| FuzzyMEn | 1.10 ± 0.20 | 0.99 ± 0.21 | <0.01 | 1.22 ± 0.13 | 1.14 ± 0.15 | <0.05 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Liu, C.; Ji, L.; Yang, J.; Liu, C. Effect of a Percutaneous Coronary Intervention Procedure on Heart Rate Variability and Pulse Transit Time Variability: A Comparison Study Based on Fuzzy Measure Entropy. Entropy 2016, 18, 246. https://0-doi-org.brum.beds.ac.uk/10.3390/e18070246
Zhang G, Liu C, Ji L, Yang J, Liu C. Effect of a Percutaneous Coronary Intervention Procedure on Heart Rate Variability and Pulse Transit Time Variability: A Comparison Study Based on Fuzzy Measure Entropy. Entropy. 2016; 18(7):246. https://0-doi-org.brum.beds.ac.uk/10.3390/e18070246
Chicago/Turabian StyleZhang, Guang, Chengyu Liu, Lizhen Ji, Jing Yang, and Changchun Liu. 2016. "Effect of a Percutaneous Coronary Intervention Procedure on Heart Rate Variability and Pulse Transit Time Variability: A Comparison Study Based on Fuzzy Measure Entropy" Entropy 18, no. 7: 246. https://0-doi-org.brum.beds.ac.uk/10.3390/e18070246