Polyanionic Drugs and Viral Oncogenesis: a Novel Approach to Control Infection, Tumor-associated Inflammation and Angiogenesis
Abstract
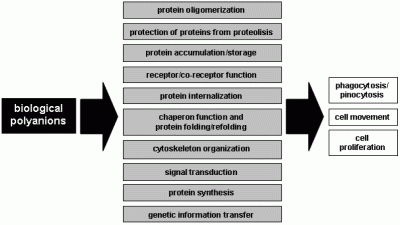
:Introduction
- i)
- in biological fluids and at the cell surface, large polyanions such as glycosaminoglycans (GAGs) bring proteins together to favour protein-protein interactions [1].
- ii)
- GAGs and heparan sulfate proteoglycans (HSPGs) of the extracellular matrix (ECM) act as a storage site for various proteins. They also protect bound proteins from degradation, prolong their lifespan and regulate their bioavailability [2].
- iii)
- iv)
- v)
- vi)
- the polyanionic nature of many intracellular second messengers plays a major role in their biology (i.e. inositol phosphate). Each event of phosphorylation results in a gain of two negative charges, and the degree of phosphorylation can be quite extensive [8]. This conveys to phosphorylated proteins a polyanionic feature with consequent “docking” properties that allow the binding and activation of other second messengers.
- vii)
- the phosphorylated form of tubulin and actin (see above) and nucleic acids are intracellular polyanions that play essential roles in cytoskeleton organization, cell division, DNA transcription and protein synthesis.
| compartment | polyanions |
|---|---|
| extracellular environment (biologic fluids, extracellular matrix) | free GAGs and GSLs, proteoglycans |
| cell membrane | membrane-associated proteoglycans and GSLs, NeuAc-bearing glycoproteins |
| intracellular environment (different compartments/organules) | GAGs, proteoglycans, GSLs, RNA, DNA, ribosomes, phosphorylated proteins, actin, microtubules |


Polyanionic compounds and infectious diseases
- i)
- extracellularly, PS/PSN compete with HSPGs for the binding to the main determinants of virus infectivity such as the human immunodeficiency virus (HIV) gp120 glycoprotein [33,34,35,36]. The degree of sulfation as well as the disposition of sulfated groups of the saccharidic chain of HSPGs seems to be of particular importance for their capacity to interact with viral proteins. One of the better characterized case is that of herpes virus, whose glycoproytein gD needs to bind specifically to 3-O-sulfated glucosamines to allow virus binding and entry into target cells [37].
- ii)
- alternatively, PS/PSN bind and mask entry receptors for viruses such as the HIV receptor CD4 [38].
- iii)
- iv)
- natural PS and nutraceuticals enhance inflammatory and immunitary responses to viruses and bacteria with still unknown mechanisms. Heparin and heparan sulfate (HS) increase cytotoxic T lymphocytes responses and production of cytokines [45]. Sulfatides trigger TNF-α and CXCL8 overexpression in neutrophils [46]. Sulfated polysaccharides from Grifola frondosa [47] increase proliferation and tumoricidal activity of lymphocytes and macrophages. In these latter cells, exopolysaccharide from marine microalga Gyrodinium impudicum [48] increases phagocytosis, lysosomal enzyme activity, production of nitrite, H2O2, TNF-α and IL-6.
- v)
- virokines are virally encoded proteins secreted from infected cells that modulate different aspects of the host immune system to maintain a suitable habitat for viral replication. In addition, they often act as cytokines that contribute to cell proliferation [49]. Myxoma virus CC-chemokine inhibitor (M-T1) is a poxvirus secreted virulence factor that binds to sulfated GAGs of target cells affecting chemokines function [50], while the E163 protein from Ectromelia virus binds to the GAGs binding site of CXCL10 and CXCL12, thus inhibiting their interaction with HSPGs and consequent biological activities [51]. These examples underline the interplay existing among viral proteins, chemokines and GAGs pointing to its relevance as a target for the development of PS compounds with therapeutical value. HIV Tat can be released by infected cells, acting as a virokine that binds to HSPGs [52] and stimulates different HIV-non permissive cells, contributing to AIDS-associated pathologies such as central and peripheral neuropathies, immune suppression and tumorigenesis [44,53]. Several PS/PSN effectively bind and sequester Tat in the extracellular environment, preventing its interaction with target cells and inhibiting some of its pathological effects [44].

| PS compounds | target virus |
|---|---|
| unmodified/ chemically modified heparin/HS | DNA: HSV [54,55], CMV [56], FMDV [57], HBV [58], HCV [59], HPV [60], HHV-7 [61], HHV-8 [62], VV [63], VZV [64] RNA: HTLV [65], HIV, VSV, Sindbis [63,66], DENV, JEV [67], Tacaribe, Junin [68], RSV, influenza A [69,70] |
| chondroitin sulfate | DNA: HIV [71] |
| carrageenans | DNA: HSV, CMV, HPV [72], VV [63] RNA: HIV, Sindbis, VSV [63], DENV [73], HAV [74], CHIKV, SFV [75], Tacaribe, Junin [68] |
| xylomannan sulfate F6 | DNA: HSV, CMV [42] RNA: HIV, influenza A/B, Tacaribe, Junin [42] |
| galactan sulfate | DNA: HSV, CMV, VV [42] RNA: HIV, Sindbis, SFV, VSV, influenza A, RSV [42], DENV [76] |
| fucoidan | DNA: HSV, CMV [63] RNA: HIV, Sindbis, VSV [63], RSV [77], SFSV [78], CHIKV, SFV [75], Tacaribe, Junin [68], HTLV [79] |
| rhamnan sulfate | DNA: HSV, CMV [80] RNA: HIV [80] |
| cellulose sulfate | DNA: HSV [81], HPV [82] RNA: HIV [83] |
| dextran sulfate | DNA: HSV, CMV, HPV, VV [82], HBV [58], HHV-7 [61] RNA: HIV , Sindbis, VSV [63], RSV, influenza A, Tacaribe, Junin, SFV [42], CHIKV [75], SFSV [78], YFV [84], RV [85] HTLV [86] |
| colominic acid | RNA: HIV [87], RTV [88] |
| curdlan sulfate | DNA: CMV [89] RNA: HIV [89] |
| glyloid sulfate 4324 | RNA: RV [85] |
| PI 88 | DNA: HSV [54] RNA: DENV, JEV [67] |
| K5 derivatives | DNA: HPV [60] RNA: HIV [90] |
| PPS | DNA: HSV, CMV, HHV-7 [61], VV [42] RNA: HIV, Sindbis, VSV [63], RSV, influenza A, Tacaribe, Junin [42], SFSV [78], DENV, JEV [67] |
| polyester | RNA: HIV [91] |
| chitin derivatives | DNA: HSV [92] |
| Y-ART-4 | RNA: HIV [93] |
| PSN compound | target virus |
| suramin | DNA: HBV [94], HCV [95], HHV-8 [96], HSV [97] RNA: HTLV-1 [98]; HIV [99] |
| suramin analogs | DNA: CMV [100] RNA: HIV [101] |
| PSS | DNA: HTLV [98], HSV [81], HBV [58], HHV-7 [61], HPV [82], CMV [100] RNA: HIV [102], RSV, influenza A [69], YFV [84] |
| porphyrins | RNA: HIV [103] |
| PS compounds | target microrganism |
|---|---|
| unmodified and chemically modified heparin/HS | bacteria: Staphylococcus epidermidis [104], Staphylococcus aureus [105], Staphylococcus hemolyticus [106], Listeria monocitogenes [107], Helicobacter pylori [108], Escherichia coli [109], Borrelia burgdorferi [110], Neisseria gonorroheae, Chlamidia trachomatis [111], Mycobacterium tubercolosis [112], Burkholderia pseudomallei [113], Legionella pneumophila [114] protozoa: Leishmania amazonensis [115], Trypanosoma brucei [116], Plasmodium falciparum [117,118], Tripanosoma cruzi [119], Toxoplasma gondii [120], Giardia lamblia [121] |
| chondroitin sulfate | bacteria: Staphylococcus epidermidis, aureus, hemolyticus [106], Listeria monocitogenes [107] protozoa: Plasmodium falciparum [122], Toxoplasma gondii [120] |
| carrageenans | bacteria: Helicobacter pylori [108] protozoa: Plasmodium falciparum [123] |
| fucoidan | bacteria: Staphylococcus epidermidis, aureus, hemolyticus [106], Anaplasma phagocytophilum [124], Helicobacter pylori [125] protozoa: Cryptosporidium parvum [126], Toxoplasma gondii [127] |
| cellulose sulfate | bacteria: Gardenella vaginalis [128] protozoa: Plasmodium falciparum, Toxoplasma gondii [127] |
| dextran sulfate | bacteria: Staphylococcus epidermidis, aureus, hemolyticus [106], Helicobacter pylori [108], Borrelia burgdorferi [110], Neisseria gonorrhoeae, Chlamidia trachomatis [111], Bacillus anthracis [113], Legionella pneumophila [114] protozoa: Plasmodium falciparum [129] |
| PI 88 | protozoa: Plasmodium falciparum [129] |
| PPS | bacteria: Staphylococcus epidermidis, aureus, hemolyticus [106], Neisseria gonorrhoeae, Chlamidia trachomatis [111] protozoa: Plasmodium falciparum [129] |
| PSN compounds | target microrganism |
| suramin | protozoa: Plasmodium falciparum [130], Trypanosoma cruzi [131] |
| suramin analogs | protozoa: Plasmodium falciparum [130] |
| PSS | bacteria: Neisseria ghonorrhoeae, Chlamydia trachomatis [132], Gardenella vaginalis [128] |
Polyanionic compounds, tumor and angiogenesis

- i)
- oncogenic viruses are involved in the arise of human malignancies such as carcinomas of the cervix uteri, hepatocellular carcinomas and lymphomas [135]. Thus, dealing with viral oncogenesis, PS/PSN can exert a so early effect as to block cell infection and transformation, thus preventing the arise of the tumor.
- ii)
- PS/PSN can be used for the prevention of thromboembolic diseases that significantly contribute to the morbidity and mortality of oncological patients [136].
- iii)
- different natural PS compounds exert an anti-tumor activity acting directly on tumor cells. Heparin, HS, dextran sulfate and fucoidan induce apoptosis of hepatoma and lymphoma cells [137,138]. Various PS polysaccharides inhibit tumour metastasis by blocking tumour-derived glycosidases and proteases such as heparanases [139] and matrix metallo proteinases [140].
- iv)
- several natural PS/PSN stimulate an antitumoral immune response [47], although the mechanism(s) by which they exert this effect is not fully elucidated.
- v)
- chronic inflammation promotes tumor progression mainly because pro-inflammatory cytokines suppress apoptosis and stimulate cell proliferation, angiogenesis, invasion, and metastasis [141]. Interestingly, many of these tumor-promoting cytokines are heparin-binding proteins [18] that can be intercepted and blocked by PS/PSN prodrugs.
- vi)
- tyrosine kinases (TKs) are intracellular signal transducing enzymes implicated in tumor progression. Efforts have been made to develop antagonists that interfere with the interaction of TKs with their substrates. Small synthetic cell permeable PS/PSN molecules can be internalized by cells [41], suggesting their use as intracellular antagonists of cytoplasmic TKs involved in oncogenesis.
- vii)
- angiogenesis, the process of new blood vessel formation from pre-existing ones, is an absolute requirement for tumor growth and metastatization [142]. Angiogenesis is mediated by angiogenic growth factors (AGFs) (Table 4) that stimulate an uncontrolled endothelial cell (EC) activation by interacting with specific TK receptors expressed on the EC surface [143]. However, to exert a full angiogenic response, some AGFs must interact also with EC surface NeuAc-bearing gangliosides [3,18] and HSPGs [18]. In effect, almost all the AGFs are heparin-binding proteins and may as well bind to GLSs [18]. A wide array of PS/PSN exert a potent antiangiogenic effect in vitro and in vivo by binding and sequestering AGFs in the extracellular environment, thus preventing their action on ECs (Table 4). The same effect can be exerted by free GLSs [18,144,145].
| polysulfated compounds | target AGF |
|---|---|
| unmodified and chemically modified heparin/HS | VEGF [146,147,148,149], FGF2 [2,150,151,152], HGF, PDGF [153,154,155], Tat [1,156,157], midkine [158], angiogenin [159], angiopoietin [160], pleiotrophin [161] |
| chondroitin sulfate | FGF2 [150], PDGF [162], midkine [158], peliotrophin [161] |
| oligosaccharides from alginic acid of seaweed | VEGF [163] |
| polysaccharides from Antrodia cinnamomea | VEGF [154] |
| carrageenans | FGF2 [164,165] |
| fucoidan | VEGF, FGF2 [166,167] |
| SargA (from Sargassum Stenofillum) | FGF2 [168] |
| dermatan sulfate | FGF2 [150], HGF [169] |
| laminarin sulfate | FGF2 [170] |
| SPMG | FGF2 [171], Tat [172] |
| sulfatides | FGF2 [144], HGF [173], midkine [174] |
| dextran sulfate | VEGF, FGF2 [175,176], HGF [177] |
| exopolysaccharide from Alteromonas infernos | VEGF, FGF2 [178] |
| heparin-carrying polystyrene | VEGF, FGF2, HGF [179] |
| heparin oligomer glycodendrimers | FGF2 [180] |
| heparin-mimicking sulfated peptides | VEGF [181] |
| suleparoide (HS analog) | FGF2 [182] |
| K5 derivatives | FGF2 [183,184], Tat [185] |
| PI-88 and analogs | VEGF, FGF2 [186,187] |
| RGTAs (synthetic GAG) | VEGF [188], FGF2 [189] |
| sucrose octasulfate | FGF2 [190] |
| beta-(1->4)-galacto oligosaccharides | FGF2 [191] |
| PPS | FGF2 [192], Tat [193] |
| β-cyclodextrin | FGF2 [194], Tat [195] |
| PSN compounds | target AGF |
| suramin | VEGF [196], FGF2 [197,198] |
| suramin analogs | VEGF, PDGF [199], FGF2 [200], Tat [29,201] |
| PSS | FGF2 [202], Tat [203] |
Polyanionic compounds as drugs: drawbacks and perspectives
- i)
- some viruses are endowed with a well known transforming capability, while some bacterial infections are known to favour the arise of tumors.
- ii)
- some virokines and bacterial toxins play a role in the development of tumors.
- iii)
- infectious diseases trigger inflammation that, in turn, triggers neovascularization, a process that is an absolute requirement for tumor growth and metastatization.
- i)
- PS/PSN such as PPS [40,193,206,207,208], suramin and analogs [29,209,210] and synthetic sulfonic acid polymers [203,211,212] are able to simultaneously bind and mask gp120 (thus inhibiting HIV infection), neutralize intracellular enzymes (such as reverse transcriptase and integrase) and inhibit the extracellular form of Tat (implicated in several AIDS-associated pathologies [213]). These observations suggest the possibility (and the opportunity) to design and produce polyanionic drugs able to bind different viral proteins simultaneously, thus interfering at once with different steps of the virus cycle (Figure 3). This “multivalent” binding capacity may limit the arise of drug resistant viral strains that, to date, represents the major limit of common antiviral therapies aimed to a single molecular target.
- ii)
- in advanced stages of human tumors, usually characterized by a high degree of vascularization, different AGFs are expressed at high levels at the same time, suggesting that tumor neovascularization is often the result of the simultaneous action of different AGFs [214]. Thus, the possibility to efficiently inhibit neovascularization in vivo by using an inhibitor specifically directed against a single AGF is far-off [214], while “multivalent” polyanionic drugs (able to bind different AGFs) may be more effective in inhibiting angiogenesis and consequent tumor progression in vivo.
- i)
- ii)
| Feature | HSPGs | GSLs |
|---|---|---|
| capacity to bind multiple proteins (via their negatively charged carboxyl or sulfated groups, respectively) | [18] | [18] |
| protection of bound proteins from proteolitic degradation | [2] | [144] |
| receptor/coreceptor function for AGFs and tumor growth factors (when cell membrane associated) | [2] | [3] |
| entry receptor for viruses (when cell membrane associated) | [32] | [6] |
| mobilization/shedding from cell membrane in the body fluids | [15] | [2] |
| antagonist activity (when in their soluble form) | [2] | [144] |
| chaperone function (when in their intracellular form) | [7] | [217] |

Acknowledgements
References and Notes
- Rusnati, M.; Tulipano, G.; Spillmann, D.; Tanghetti, E.; Oreste, P.; Zoppetti, G.; Giacca, M.; Presta, M. Multiple interactions of HIV-I Tat protein with size-defined heparin oligosaccharides. J. Biol. Chem. 1999, 274, 28198–28205. [Google Scholar] [CrossRef]
- Rusnati, M.; Presta, M. Interaction of angiogenic basic fibroblast growth factor with endothelial cell heparan sulfate proteoglycans. Biological implications in neovascularization. Int. J. Clin. Lab. Res. 1996, 26, 15–23. [Google Scholar] [CrossRef]
- Rusnati, M.; Urbinati, C.; Tanghetti, E.; Dell'Era, P.; Lortat-Jacob, H.; Presta, M. Cell membrane GM1 ganglioside is a functional coreceptor for fibroblast growth factor 2. Proc. Natl. Acad. Sci. USA 2002, 99, 4367–4372. [Google Scholar] [CrossRef]
- Poon, G. M.; Gariepy, J. Cell-surface proteoglycans as molecular portals for cationic peptide and polymer entry into cells. Biochem. Soc. Trans. 2007, 35, 788–793. [Google Scholar] [CrossRef]
- Olofsson, S.; Bergstrom, T. Glycoconjugate glycans as viral receptors. Ann. Med. 2005, 37, 154–172. [Google Scholar] [CrossRef]
- Wadstrom, T.; Ljungh, A. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J. Med. Microbiol. 1999, 48, 223–233. [Google Scholar] [CrossRef]
- Rusnati, M.; Urbinati, C.; Presta, M. Internalization of basic fibroblast growth factor (bFGF) in cultured endothelial cells: role of the low affinity heparin-like bFGF receptors. J. Cell Physiol. 1993, 154, 152–161. [Google Scholar] [CrossRef]
- Jones, L. S.; Yazzie, B.; Middaugh, C. R. Polyanions and the proteome. Mol. Cell Proteomics 2004, 3, 746–769. [Google Scholar] [CrossRef]
- Salamat-Miller, N.; Fang, J.; Seidel, C. W.; Assenov, Y.; Albrecht, M.; Middaugh, C. R. A network-based analysis of polyanion-binding proteins utilizing human protein arrays. J. Biol. Chem. 2007, 282, 10153–10163. [Google Scholar]
- Doiron, A. L.; Kirkpatrick, A. P.; Rinker, K. D. TGF-beta and TNF-a affect cell surface proteoglycan and sialic acid expression on vascular endothelial cells. Biomed. Sci. Instrum. 2004, 40, 331–336. [Google Scholar]
- Hippenmeyer, P. J.; Ruminski, P. G.; Rico, J. G.; Lu, H. S.; Griggs, D. W. Adenovirus inhibition by peptidomimetic integrin antagonists. Antiviral. Res. 2002, 55, 169–178. [Google Scholar] [CrossRef]
- Guerrero, C. A.; Mendez, E.; Zarate, S.; Isa, P.; Lopez, S.; Arias, C. F. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc. Natl. Acad. Sci. USA 2000, 97, 14644–14649. [Google Scholar] [CrossRef]
- Schengrund, C. L. "Multivalent" saccharides: development of new approaches for inhibiting the effects of glycosphingolipids-binding pathogens. Biochem. Pharm. 2003, 65, 699–707. [Google Scholar] [CrossRef]
- Gullino, P. Prostaglandins and gangliosides of tumor microenvironment: their role in angiogenesis. Acta Oncol. 1995, 34, 439–441. [Google Scholar] [CrossRef]
- Chang, F.; Li, R.; Ladisch, S. Shedding of gangliosides by human medulloblastoma cells. Exp. Cell Res. 1997, 234, 341–346. [Google Scholar] [CrossRef]
- Kumar, C. C. Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr. Drug Targets 2003, 4, 123–131. [Google Scholar] [CrossRef]
- Munoz, E. M.; Linhardt, R. J. Heparin-binding domains in vascular biology. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1549–1557. [Google Scholar] [CrossRef]
- Rusnati, M.; Presta, M. Extracellular angiogenic growth factor interactions: an angiogenesis interactome survey. Endothelium 2006, 13, 93–111. [Google Scholar] [CrossRef]
- Powell, A. K.; Yates, E. A.; Fernig, D. G.; Turnbull, J. E. Interactions of heparin/heparan sulfate with proteins: appraisal of structural factors and experimental approaches. Glycobiology 2004, 14, 17R–30R. [Google Scholar] [CrossRef]
- Rusnati, M.; Urbinati, C.; Presta, M. Glycomics and angiogenesis: biological implications and therapeutical exploiting. In New Development in Therapeutic Glycomics; Delehedde, M., Lortat-Jacob, H., Eds.; Research Signpost: Trivandum, India, 2006; pp. 33–77. [Google Scholar]
- Petitou, M.; Casu, B.; Lindahl, U. 1976-1983, a critical period in the history of heparin: the discovery of the antithrombin binding site. Biochimie 2003, 85, 83–89. [Google Scholar] [CrossRef]
- Conrad, H. E. Heparin binding proteins; Academic Press: New York, USA, 1998. [Google Scholar]
- Lindahl, U.; Lidholt, K.; Spillmann, D.; Kjellen, L. More to "heparin" than anticoagulation. Thromb. Res. 1994, 75, 1–32. [Google Scholar] [CrossRef]
- Casu, B.; Grazioli, G.; Razi, N.; Guerrini, M.; Naggi, A.; Torri, G.; Oreste, P.; Tursi, F.; Zoppetti, G.; Lindahl, U. Heparin-like compounds prepared by chemical modification of capsular polysaccharide from E. coli K5. Carbohydr. Res. 1994, 263, 271–284. [Google Scholar] [CrossRef]
- Volpi, N. Therapeutic applications of glycosaminoglycans. Curr. Med. Chem. 2006, 13, 1799–1810. [Google Scholar] [CrossRef]
- Rusnati, M.; Presta, M. Fibroblast growth factors/fibroblast growth factor receptors as targets for the development of anti-angiogenesis strategies. Curr. Pharm. Des. 2007, 13, 2025–2044. [Google Scholar] [CrossRef]
- Barrett, M. P.; Gilbert, I. H. Targeting of toxic compounds to the trypanosome's interior. Adv. Parasitol. 2006, 63, 125–183. [Google Scholar] [CrossRef]
- Abiose, A. Onchocercal eye disease and the impact of Mectizan treatment. Ann. Trop. Med. Parasitol. 1998, 92 Suppl. 1, S11–S22. [Google Scholar] [CrossRef]
- Rusnati, M.; Tulipano, G.; Urbinati, C.; Tanghetti, E.; Giuliani, R.; Giacca, M.; Ciomei, M.; Corallini, A.; Presta, M. The basic domain in HIV-1 Tat protein as a target for polysulfonated heparin-mimicking extracellular Tat antagonists. J. Biol. Chem. 1998, 273, 16027–16037. [Google Scholar] [CrossRef]
- Manetti, F.; Corelli, F.; Mongelli, N.; Borgia, A. L.; Botta, M. Research on anti-HIV-1 agents. Investigation on the CD4-Suradista binding mode through docking experiments. J. Comput. Aided Mol. Des. 2000, 14, 355–368. [Google Scholar] [CrossRef]
- Finch, P. W.; Yee, L. K.; Chu, M. Y.; Chen, T. M.; Lipsky, M. H.; Maciag, T.; Friedman, S.; Epstein, M. H.; Calabresi, P. Inhibition of growth factor mitogenicity and growth of tumor cell xenografts by a sulfonated distamycin A derivative. Pharmacology 1997, 55, 269–278. [Google Scholar] [CrossRef]
- Spillmann, D. Heparan sulfate: anchor for viral intruders? Biochimie 2001, 83, 811–817. [Google Scholar] [CrossRef]
- Barbouche, R.; Lortat-Jacob, H.; Jones, I. M.; Fenouillet, E. Glycosaminoglycans and protein disulfide isomerase-mediated reduction of HIV Env. Mol. Pharmacol. 2005, 67, 1111–1118. [Google Scholar] [CrossRef]
- Vives, R. R.; Imberty, A.; Sattentau, Q. J.; Lortat-Jacob, H. Heparan sulfate targets the HIV-1 envelope glycoprotein gp120 coreceptor binding site. J. Biol. Chem. 2005, 280, 21353–21357. [Google Scholar] [CrossRef]
- Harrop, H. A.; Rider, C. C. Heparin and its derivatives bind to HIV-1 recombinant envelope glycoproteins, rather than to recombinant HIV-1 receptor, CD4. Glycobiology 1998, 8, 131–137. [Google Scholar] [CrossRef]
- Crublet, E.; Andrieu, J. P.; Vives, R. R.; Lortat-Jacob, H. The HIV-1 envelope glycoprotein gp120 features four heparan sulfate binding domains, including the co-receptor binding site. J. Biol. Chem. 2008, 283, 15193–15200. [Google Scholar]
- Shukla, D.; Liu, J.; Blaiklock, P.; Shworak, N. W.; Bai, X.; Esko, J. D.; Cohen, G. H.; Eisenberg, R. J.; Rosenberg, R. D.; Spear, P. G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 1999, 99, 13–22. [Google Scholar] [CrossRef]
- Lederman, S.; Gulick, R.; Chess, L. Dextran sulfate and heparin interact with CD4 molecules to inhibit the binding of coat protein (gp120) of HIV. J. Immunol. 1989, 143, 1149–1154. [Google Scholar]
- Ueki, M.; Watanabe, S.; Saitoh, T.; Nakashima, H.; Yamamoto, N.; Ogawara, H. Synthesis and chain length-anti-HIV activity relationship of fully N- and O-sulfated homooligomers of tyrosine. Bioorg. Med. Chem. 2001, 9, 487–492. [Google Scholar] [CrossRef]
- Sydow, G.; Klocking, H. P. Effect of pentosan polysulfate (SP 54) on the reverse transcriptase activity of several retroviruses. Biomed. Biochim. Acta 1987, 46, 527–530. [Google Scholar]
- Rideout, D.; Schinazi, R.; Pauza, C. D.; Lovelace, K.; Chiang, L. C.; Calogeropoulou, T.; McCarthy, M.; Elder, J. H. Derivatives of 4-amino-3,6-disulfonato-1,8-naphthalimide inhibit reverse transcriptase and suppress human and feline immunodeficiency virus expression in cultured cells. J. Cell. Biochem. 1993, 51, 446–457. [Google Scholar]
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Moelling, K.; Schulze, T.; Diringer, H. Inhibition of human immunodeficiency virus type 1 RNase H by sulfated polyanions. J. Virol. 1989, 63, 5489–5491. [Google Scholar]
- Rusnati, M.; Presta, M. HIV-1 Tat protein: a target for the development of anti-AIDS therapies. Drug Fut. 2002, 27, 481–493. [Google Scholar] [CrossRef]
- Dziarski, R. Synergistic enhancement of T cell responses and interleukin-1 receptor expression by interleukin-1 and heparin or dextran sulfate. Cell Immunol. 1992, 145, 100–110. [Google Scholar] [CrossRef]
- Laudanna, C.; Constantin, G.; Baron, P.; Scarpini, E.; Scarlato, G.; Cabrini, G.; Dechecchi, C.; Rossi, F.; Cassatella, M. A.; Berton, G. Sulfatides trigger increase of cytosolic free calcium and enhanced expression of tumor necrosis factor-alpha and interleukin-8 mRNA in human neutrophils. Evidence for a role of L-selectin as a signaling molecule. J. Biol. Chem. 1994, 269, 4021–4026. [Google Scholar]
- Nie, X.; Shi, B.; Ding, Y.; Tao, W. Preparation of a chemically sulfated polysaccharide derived from Grifola frondosa and its potential biological activities. Int. J. Biol. Macromol. 2006, 39, 228–233. [Google Scholar] [CrossRef]
- Bae, S. Y.; Yim, J. H.; Lee, H. K.; Pyo, S. Activation of murine peritoneal macrophages by sulfated exopolysaccharide from marine microalga Gyrodinium impudicum (strain KG03): involvement of the NF-kappaB and JNK pathway. Int. Immunopharmacol. 2006, 6, 473–484. [Google Scholar] [CrossRef]
- Smith, S. A.; Kotwal, G. J. Virokines: novel immunomodulatory agents. Expert Opin. Biol. Ther. 2001, 1, 343–357. [Google Scholar] [CrossRef]
- Seet, B. T.; Barrett, J.; Robichaud, J.; Shilton, B.; Singh, R.; McFadden, G. Glycosaminoglycan binding properties of the myxoma virus CC-chemokine inhibitor, M-T1. J. Biol. Chem. 2001, 276, 30504–30513. [Google Scholar]
- Ruiz-Arguello, M. B.; Smith, V. P.; Campanella, G. S.; Baleux, F.; Arenzana-Seisdedos, F.; Luster, A. D.; Alcami, A. An ectromelia virus protein that interacts with chemokines through their glycosaminoglycan binding domain. J. Virol. 2008, 82, 917–926. [Google Scholar] [CrossRef]
- Tyagi, M.; Rusnati, M.; Presta, M.; Giacca, M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2001, 276, 3254–3261. [Google Scholar] [CrossRef]
- Rusnati, M.; Presta, M. HIV-1 Tat protein and endothelium: from protein/cell interaction to AIDS-associated pathologies. Angiogenesis 2002, 5, 141–151. [Google Scholar] [CrossRef]
- Nyberg, K.; Ekblad, M.; Bergstrom, T.; Freeman, C.; Parish, C. R.; Ferro, V.; Trybala, E. The low molecular weight heparan sulfate-mimetic, PI-88, inhibits cell-to-cell spread of herpes simplex virus. Antiviral Res. 2004, 63, 15–24. [Google Scholar] [CrossRef]
- Herold, B. C.; Gerber, S. I.; Belval, B. J.; Siston, A. M.; Shulman, N. Differences in the susceptibility of herpes simplex virus types 1 and 2 to modified heparin compounds suggest serotype differences in viral entry. J. Virol. 1996, 70, 3461–3469. [Google Scholar]
- Mastromarino, P.; Seganti, L.; Petruzziello, R.; Gabrieli, R.; Divizia, M.; Pana, A.; Orsi, N. Influence of polyions on the early steps of enterovirus infection. J. Chemother. 1991, 3, 203–208. [Google Scholar]
- Sa-Carvalho, D.; Rieder, E.; Baxt, B.; Rodarte, R.; Tanuri, A.; Mason, P. W. Tissue culture adaption of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J. Virol. 1997, 71, 5115–5123. [Google Scholar]
- Ying, C.; Van Pelt, J. F.; Van Lommel, A.; Van Ranst, M.; Leyssen, P.; De Clercq, E.; Neyts, J. Sulphated and sulphonated polymers inhibit the initial interaction of hepatitis B virus with hepatocytes. Antivir. Chem. Chemother. 2002, 13, 157–164. [Google Scholar]
- Basu, A.; Kanda, T.; Beyene, A.; Saito, K.; Meyer, K.; Ray, R. Sulfated homologues of heparin inhibit hepatitis C virus entry into mammalian cells. J. Virol. 2007, 81, 3933–3941. [Google Scholar] [CrossRef]
- Lembo, D.; Donalisio, M.; Rusnati, M.; Bugatti, A.; Cornaglia, M.; Cappello, P.; Giovarelli, M.; Oreste, P.; Landolfo, S. Sulfated K5 Escherichia coli polysaccharide derivatives as wide range inhibitors of genital types of human papillomavirus. Antimicrob. Agents Chemother. 2008, 52, 1374–1381. [Google Scholar] [CrossRef]
- Zhang, Y.; Schols, D.; De Clercq, E. Selective activity of various antiviral compounds against HHV-7 infection. Antiviral. Res. 1999, 43, 23–35. [Google Scholar] [CrossRef]
- Birkmann, A.; Mahr, K.; Ensser, A.; Yaguboglu, S.; Titgemeyer, F.; Fleckenstein, B.; Neipel, F. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J. Virol. 2001, 75, 11583–11593. [Google Scholar] [CrossRef]
- Baba, M.; Snoeck, R.; Pauwels, R.; de Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef]
- Zhu, Z.; Gershon, M. D.; Ambron, R.; Gabel, C.; Gershon, A. A. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose-6-phosphate and heparin. Proc. Natl. Acad. Sci. USA 1995, 92, 3546–2550. [Google Scholar] [CrossRef]
- Jones, K. S.; Petrow-Sadowski, C.; Bertolette, D. C.; Huang, Y.; Ruscetti, F. W. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 2005, 79, 12692–12702. [Google Scholar]
- Lopalco, L.; Ciccomascolo, F.; Lanza, P.; Zoppetti, G.; Caramazza, I.; Leoni, F.; Beretta, A.; Siccardi, A. G. Anti-HIV type 1 properties of chemically modified heparins with diminished anticoagulant activity. AIDS Res. Hum. Retroviruses 1994, 10, 787–793. [Google Scholar]
- Lee, E.; Pavy, M.; Young, N.; Freeman, C.; Lobigs, M. Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic flaviviruses. Antiviral. Res. 2006, 69, 31–38. [Google Scholar] [CrossRef]
- Andrei, G.; De Clercq, E. Inhibitory effect of selected antiviral compounds on arenavirus replication in vitro. Antiviral Res. 1990, 14, 287–299. [Google Scholar] [CrossRef]
- Hosoya, M.; Balzarini, J.; Shigeta, S.; De Clercq, E. Differential inhibitory effects of sulfated polysaccharides and polymers on the replication of various myxoviruses and retroviruses, depending on the composition of the target amino acid sequences of the viral envelope glycoproteins. Antimicrob. Agents Chemother. 1991, 35, 2515–2520. [Google Scholar] [CrossRef]
- Hallak, L. K.; Spillmann, D.; Collins, P. L.; Peeples, M. E. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J. Virol. 2000, 74, 10508–10513. [Google Scholar]
- Konlee, M. Sulfated polysaccharides (chondroitin sulfate and carrageenan) plus glucosamine sulfate are potent inhibitors of HIV. Posit. Health News 1998, 4–7. [Google Scholar]
- Buck, C. B.; Thompson, C. D.; Roberts, J. N.; Muller, M.; Lowy, D. R.; Schiller, J. T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar]
- Talarico, L. B.; Damonte, E. B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar]
- Girond, S.; Crance, J. M.; Van Cuyck-Gandre, H.; Renaudet, J.; Deloince, R. Antiviral activity of carrageenan on hepatitis A virus replication in cell culture. Res. Virol. 1991, 142, 261–270. [Google Scholar] [CrossRef]
- Briolant, S.; Garin, D.; Scaramozzino, N.; Jouan, A.; Crance, J. M. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination. Antiviral Res. 2004, 61, 111–117. [Google Scholar]
- Talarico, L. B.; Pujol, C. A.; Zibetti, R. G.; Faria, P. C.; Noseda, M. D.; Duarte, M. E.; Damonte, E. B. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res. 2005, 66, 103–110. [Google Scholar]
- Malhotra, R.; Ward, M.; Bright, H.; Priest, R.; Foster, M. R.; Hurle, M.; Blair, E.; Bird, M. Isolation and characterisation of potential respiratory syncytial virus receptor(s) on epithelial cells. Microbes Infect. 2003, 5, 123–133. [Google Scholar] [CrossRef]
- Crance, J. M.; Gratier, D.; Guimet, J.; Jouan, A. Inhibition of sandfly fever Sicilian virus (Phlebovirus) replication in vitro by antiviral compounds. Res. Virol. 1997, 148, 353–365. [Google Scholar] [CrossRef]
- Romanos, M. T.; Andrada-Serpa, M. J.; Mourao, P. A.; Yoneshigue-Valentin, Y.; Costa, S. S.; Pereira, M. S.; Miranda, M. M.; Goncalves, J. L.; Wigg, M. D. A sulphated fucan from the Laminaria abyssalis inhibits the human T cell lymphotropic virus type 1-induced syncytium formation in HeLa cells. Antivir. Chem. Chemother. 2002, 13, 219–221. [Google Scholar]
- Flores, O.; Lee, G.; Kessler, J.; Miller, M.; Schlief, W.; Tomassini, J.; Hazuda, D. Host-cell positive transcription elongation factor b kinase activity is essential and limiting for HIV type 1 replication. Proc. Natl. Acad. Sci. USA 1999, 96, 7208–7213. [Google Scholar]
- Cheshenko, N.; Keller, M. J.; MasCasullo, V.; Jarvis, G. A.; Cheng, H.; John, M.; Li, J. H.; Hogarty, K.; Anderson, R. A.; Waller, D. P.; Zaneveld, L. J.; Profy, A. T.; Klotman, M. E.; Herold, B. C. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob. Agents Chemother. 2004, 48, 2025–2036. [Google Scholar] [CrossRef]
- Christensen, N. D.; Reed, C. A.; Culp, T. D.; Hermonat, P. L.; Howett, M. K.; Anderson, R. A.; Zaneveld, L. J. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob. Agents Chemother. 2001, 45, 3427–3432. [Google Scholar] [CrossRef]
- El-Sadr, W. M.; Mayer, K. H.; Maslankowski, L.; Hoesley, C.; Justman, J.; Gai, F.; Mauck, C.; Absalon, J.; Morrow, K.; Masse, B.; Soto-Torres, L.; Kwiecien, A. Safety and acceptability of cellulose sulfate as a vaginal microbicide in HIV-infected women. Aids 2006, 20, 1109–1116. [Google Scholar] [CrossRef]
- Neyts, J.; Meerbach, A.; McKenna, P.; De Clercq, E. Use of the yellow fever virus vaccine strain 17D for the study of strategies for the treatment of yellow fever virus infections. Antiviral Res. 1996, 30, 125–132. [Google Scholar] [CrossRef]
- Mastromarino, P.; Petruzziello, R.; Macchia, S.; Rieti, S.; Nicoletti, R.; Orsi, N. Antiviral activity of natural and semisynthetic polysaccharides on the early steps of rubella virus infection. J. Antimicrob. Chemother. 1997, 39, 339–345. [Google Scholar]
- Ida, H.; Kurata, A.; Eguchi, K.; Yamashita, I.; Nakashima, M.; Sakai, M.; Kawabe, Y.; Nakamura, T.; Nagataki, S. Mechanism of inhibitory effect of dextran sulfate and heparin on human T-cell lymphotropic virus type I (HTLV-I)-induced syncytium formation in vitro: role of cell-to-cell contact. Antiviral Res. 1994, 23, 143–159. [Google Scholar]
- Yang, D. W.; Ohta, Y.; Yamaguchi, S.; Tsukada, Y.; Haraguchi, Y.; Hoshino, H.; Amagai, H.; Kobayashi, I. Sulfated colominic acid: an antiviral agent that inhibits the human immunodeficiency virus type 1 in vitro. Antiviral Res. 1996, 31, 95–104. [Google Scholar] [CrossRef]
- Konishi, K.; Gu, Y.; Hatano, I.; Ushijima, H. Effect of sulfated colominic acid on enteric virus (rotavirus, poliovirus and coxsackievirus) infections in vitro. Jpn. J. Infect. Dis. 2000, 53, 62–66. [Google Scholar]
- Gordon, M.; Deeks, S.; De Marzo, C.; Goodgame, J.; Guralnik, M.; Lang, W.; Mimura, T.; Pearce, D.; Kaneko, Y. Curdlan sulfate (CRDS) in a 21-day intravenous tolerance study in human immunodeficiency virus (HIV) and cytomegalovirus (CMV) infected patients: indication of anti-CMV activity with low toxicity. J. Med. 1997, 28, 108–128. [Google Scholar]
- Vicenzi, E.; Gatti, A.; Ghezzi, S.; Oreste, P.; Zoppetti, G.; Poli, G. Broad spectrum inhibition of HIV-1 infection by sulfated K5 Escherichia coli polysaccharide derivatives. Aids 2003, 17, 177–181. [Google Scholar] [CrossRef]
- Lederman, S.; Bergmann, J. E.; Cleary, A. M.; Yellin, M. J.; Fusco, P. J.; Chess, L. Sulfated polyester interactions with the CD4 molecule and with the third variable loop domain (v3) of gp120 are chemically distinct. AIDS Res. Hum. Retroviruses 1992, 8, 1599–1610. [Google Scholar] [CrossRef]
- Ishihara, C.; Yoshimatsu, K.; Tsuji, M.; Arikawa, J.; Saiki, I.; Tokura, S.; Azuma, I. Anti-viral activity of sulfated chitin derivatives against Friend murine leukaemia and herpes simplex type-1 viruses. Vaccine 1993, 11, 670–674. [Google Scholar] [CrossRef]
- Ueki, M.; Watanabe, S.; Ishii, Y.; Okunaka, O.; Uchino, K.; Saitoh, T.; Higashi, K.; Nakashima, H.; Yamamoto, N.; Ogawara, H. Synthesis and anti-HIV activity of nonatyrosine N- and O1-9-decasulfate. Bioorg. Med. Chem. 2001, 9, 477–486. [Google Scholar] [CrossRef]
- Schulze, A.; Gripon, P.; Urban, S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 2007, 46, 1759–1768. [Google Scholar] [CrossRef]
- Garson, J. A.; Lubach, D.; Passas, J.; Whitby, K.; Grant, P. R. Suramin blocks hepatitis C binding to human hepatoma cells in vitro. J. Med. Virol. 1999, 57, 238–242. [Google Scholar] [CrossRef]
- Inoue, N.; Winter, J.; Lal, R. B.; Offermann, M. K.; Koyano, S. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J. Virol. 2003, 77, 8147–8152. [Google Scholar] [CrossRef]
- Aguilar, J. S.; Rice, M.; Wagner, E. K. The polysulfonated compound suramin blocks adsorption and lateral difusion of herpes simplex virus type-1 in vero cells. Virology 1999, 258, 141–151. [Google Scholar] [CrossRef]
- Pesce, C. D.; Ciprani, F.; D'Onofrio, C.; Alvino, E.; Perno, C. F.; Bonmassar, E.; Calio, R. Low concentrations of suramin can reduce in vitro infection of human cord blood lymphocytes with HTLV-I during long-term culture. Antiviral Res. 1987, 8, 247–260. [Google Scholar] [CrossRef]
- Clanton, D. J.; Buckheit, R. W., Jr.; Terpening, S. J.; Kiser, R.; Mongelli, N.; Borgia, A. L.; Schultz, R.; Narayanan, V.; Bader, J. P.; Rice, W. G. Novel sulfonated and phosphonated analogs of distamycin which inhibit the replication of HIV. Antiviral Res. 1995, 27, 335–354. [Google Scholar] [CrossRef]
- Baba, M.; Konno, K.; Shigeta, S.; Wickramasinghe, A.; Mohan, P. Selective inhibition of human cytomegalovirus replication by naphthalenedisulfonic acid derivatives. Antiviral Res. 1993, 20, 223–233. [Google Scholar] [CrossRef]
- Dezube, B. J.; Dahl, T. A.; Wong, T. K.; Chapman, B.; Ono, M.; Yamaguchi, N.; Gillies, S. D.; Chen, L. B.; Crumpacker, C. S. A fusion inhibitor (FP-21399) for the treatment of human immunodeficiency virus infection: a phase I study. J. Infect. Dis. 2000, 182, 607–610. [Google Scholar] [CrossRef]
- Taylor, D. L.; Brennan, T. M.; Bridges, C. G.; Mullins, M. J.; Tyms, A. S.; Jackson, R.; Cardin, A. D. Potent inhibition of human immunodeficiency virus by MDL 101028, a novel sulphonic acid polymer. Antiviral Res. 1995, 28, 159–173. [Google Scholar] [CrossRef]
- Vzorov, A. N.; Bozja, J.; Dixon, D. W.; Marzilli, L. G.; Compans, R. W. Parameters of inhibition of HIV-1 infection by small anionic microbicides. Antiviral Res. 2007, 73, 60–68. [Google Scholar] [CrossRef]
- Arciola, C. R.; Bustanji, Y.; Conti, M.; Campoccia, D.; Baldassarri, L.; Samori, B.; Montanaro, L. Staphylococcus epidermidis-fibronectin binding and its inhibition by heparin. Biomaterials 2003, 24, 3013–3019. [Google Scholar] [CrossRef]
- Hess, D. J.; Henry-Stanley, M. J.; Erlandsen, S. L.; Wells, C. L. Heparan sulfate proteoglycans mediate Staphylococcus aureus interactions with intestinal epithelium. Med. Microbiol. Immunol. 2006, 195, 133–141. [Google Scholar] [CrossRef]
- Fallgren, C.; Andersson, A.; Ljungh, A. The role of glycosaminoglycan binding of staphylococci in attachment to eukaryotic host cells. Curr. Microbiol. 2001, 43, 57–63. [Google Scholar] [CrossRef]
- Henry-Stanley, M. J.; Hess, D. J.; Erickson, E. A.; Garni, R. M.; Wells, C. L. Role of heparan sulfate in interactions of Listeria monocytogenes with enterocytes. Med. Microbiol. Immunol. 2003, 192, 107–115. [Google Scholar]
- Utt, M.; Wadstrom, T. Identification of heparan sulphate binding surface proteins of Helicobacter pylori: inhibition of heparan sulphate binding with sulphated carbohydrate polymers. J. Med. Microbiol. 1997, 46, 541–546. [Google Scholar] [CrossRef]
- Rosett, W.; Hodges, G. R. Antimicrobial activity of heparin. J. Clin. Microbiol. 1980, 11, 30–34. [Google Scholar]
- Leong, J. M.; Morrissey, P. E.; Ortega-Barria, E.; Pereira, M. E.; Coburn, J. Hemagglutination and proteoglycan binding by the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 1995, 63, 874–883. [Google Scholar]
- Herold, B. C.; Siston, A.; Bremer, J.; Kirkpatrick, R.; Wilbanks, G.; Fugedi, P.; Peto, C.; Cooper, M. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 1997, 41, 2776–2780. [Google Scholar]
- Menozzi, F. D.; Rouse, J. H.; Alavi, M.; Laude-Sharp, M.; Muller, J.; Bischoff, R.; Brennan, M. J.; Locht, C. Identification of a heparin-binding hemagglutinin present in mycobacteria. J. Exp. Med. 1996, 184, 993–1001. [Google Scholar] [CrossRef]
- Thomas, R.; Brooks, T. Common oligosaccharide moieties inhibit the adherence of typical and atypical respiratory pathogens. J. Med. Microbiol. 2004, 53, 833–840. [Google Scholar] [CrossRef]
- Thomas, R. J.; Brooks, T. J. Oligosaccharide receptor mimics inhibit Legionella pneumophila attachment to human respiratory epithelial cells. Microb. Pathog. 2004, 36, 83–92. [Google Scholar] [CrossRef]
- Bosetto, M. C.; Giorgio, S. Leishmania amazonensis: multiple receptor-ligand interactions are involved in amastigote infection of human dendritic cells. Exp. Parasitol. 2007, 116, 306–310. [Google Scholar] [CrossRef]
- Nishimura, K.; Shima, K.; Asakura, M.; Ohnishi, Y.; Yamasaki, S. Effects of heparin administration on Trypanosoma brucei gambiense infection in rats. J. Parasitol. 2005, 91, 219–222. [Google Scholar] [CrossRef]
- Vogt, A. M.; Pettersson, F.; Moll, K.; Jonsson, C.; Normark, J.; Ribacke, U.; Egwang, T. G.; Ekre, H. P.; Spillmann, D.; Chen, Q.; Wahlgren, M. Release of sequestered malaria parasites upon injection of a glycosaminoglycan. PLoS Pathog. 2006, 2, e100. [Google Scholar] [CrossRef]
- Schwartz-Albiez, R.; Adams, Y.; von der Lieth, C. W.; Mischnick, P.; Andrews, K. T.; Kirschfink, M. Regioselectively modified sulfated cellulose as prospective drug for treatment of malaria tropica. Glycoconj. J. 2007, 24, 57–65. [Google Scholar]
- Calvet, C. M.; Toma, L.; De Souza, F. R.; Meirelles Mde, N.; Pereira, M. C. Heparan sulfate proteoglycans mediate the invasion of cardiomyocytes by Trypanosoma cruzi. J. Eukaryot. Microbiol. 2003, 50, 97–103. [Google Scholar] [CrossRef]
- Carruthers, V. B.; Hakansson, S.; Giddings, O. K.; Sibley, L. D. Toxoplasma gondii uses sulfated proteoglycans for substrate and host cell attachment. Infect. Immun. 2000, 68, 4005–4011. [Google Scholar] [CrossRef]
- Weiland, M. E.; Palm, J. E.; Griffiths, W. J.; McCaffery, J. M.; Svard, S. G. Characterisation of alpha-1 giardin: an immunodominant Giardia lamblia annexin with glycosaminoglycan-binding activity. Int. J. Parasitol. 2003, 33, 1341–1351. [Google Scholar] [CrossRef]
- Gysin, J.; Pouvelle, B.; Fievet, N.; Scherf, A.; Lepolard, C. Ex vivo desequestration of Plasmodium falciparum-infected erythrocytes from human placenta by chondroitin sulfate A. Infect. Immun. 1999, 67, 6596–6602. [Google Scholar]
- Adams, Y.; Smith, S. L.; Schwartz-Albiez, R.; Andrews, K. T. Carrageenans inhibit the in vitro growth of Plasmodium falciparum and cytoadhesion to CD36. Parasitol. Res. 2005, 97, 290–294. [Google Scholar] [CrossRef]
- Herron, M. J.; Ericson, M. E.; Kurtti, T. J.; Munderloh, U. G. The interactions of Anaplasma phagocytophilum, endothelial cells, and human neutrophils. Ann. N. Y. Acad. Sci. 2005, 1063, 374–382. [Google Scholar]
- Shibata, H.; KimuraTakagi, I.; Nagaoka, M.; Hashimoto, S.; Sawada, H.; Ueyama, S.; Yokokura, T. Inhibitory effect of Cladosiphon fucoidan on the adhesion of Helicobacter pylori to human gastric cells. J. Nutr. Sci. Vitaminol. (Tokyo) 1999, 45, 325–336. [Google Scholar]
- Maruyama, H.; Tanaka, M.; Hashimoto, M.; Inoue, M.; Sasahara, T. The suppressive effect of Mekabu fucoidan on an attachment of Cryptosporidium parvum oocysts to the intestinal epithelial cells in neonatal mice. Life Sci. 2007, 80, 775–781. [Google Scholar] [CrossRef]
- Ortega-Barria, E.; Boothroyd, J. C. A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J. Biol. Chem. 1999, 274, 1267–1276. [Google Scholar] [CrossRef]
- Simoes, J. A.; Citron, D. M.; Aroutcheva, A.; Anderson, R. A., Jr.; Chany, C. J., 2nd; Waller, D. P.; Faro, S.; Zaneveld, L. J. Two novel vaginal microbicides (polystyrene sulfonate and cellulose sulfate) inhibit Gardnerella vaginalis and anaerobes commonly associated with bacterial vaginosis. Antimicrob. Agents Chemother. 2002, 46, 2692–2695. [Google Scholar] [CrossRef]
- Adams, Y.; Freeman, C.; Schwartz-Albiez, R.; Ferro, V.; Parish, C. R.; Andrews, K. T. Inhibition of Plasmodium falciparum growth in vitro and adhesion to chondroitin-4-sulfate by the heparan sulfate mimetic PI-88 and other sulfated oligosaccharides. Antimicrob. Agents Chemother. 2006, 50, 2850–2852. [Google Scholar] [CrossRef] [Green Version]
- Fleck, S. L.; Birdsall, B.; Babon, J.; Dluzewski, A. R.; Martin, S. R.; Morgan, W. D.; Angov, E.; Kettleborough, C. A.; Feeney, J.; Blackman, M. J.; Holder, A. A. Suramin and suramin analogues inhibit merozoite surface protein-1 secondary processing and erythrocyte invasion by the malaria parasite Plasmodium falciparum. J. Biol. Chem. 2003, 278, 47670–47677. [Google Scholar]
- Bisaggio, D. F.; Adade, C. M.; Souto-Padron, T. In vitro effects of suramin on Trypanosoma cruzi. Int. J. Antimicrob. Agents 2008, 31, 282–286. [Google Scholar] [CrossRef]
- Herold, B. C.; Bourne, N.; Marcellino, D.; Kirkpatrick, R.; Strauss, D. M.; Zaneveld, L. J.; Waller, D. P.; Anderson, R. A.; Chany, C. J.; Barham, B. J.; Stanberry, L. R.; Cooper, M. D. Poly(sodium 4-styrene sulfonate): an effective candidate topical antimicrobial for the prevention of sexually transmitted diseases. J. Infect. Dis. 2000, 181, 770–773. [Google Scholar] [CrossRef]
- Rueda, R. The role of dietary gangliosides on immunity and the prevention of infection. Br. J. Nutr. 2007, 98 Suppl. 1, S68–73. [Google Scholar]
- Schwartsmann, G.; Sprinz, E.; Kalakun, L.; Yamagushi, N.; Sander, E.; Grivicich, I.; Koya, R.; Mans, D. R. Phase II study of pentosan polysulfate (PPS) in patients with AIDS-related Kaposi's sarcoma. Tumori 1996, 82, 360–363. [Google Scholar]
- Mueller, N. Overview: viral agents and cancer. Environ. Health Perspect. 1995, 103 Suppl. 8, 259–261. [Google Scholar] [CrossRef]
- Castelli, R.; Porro, F.; Tarsia, P. The heparins and cancer: review of clinical trials and biological properties. Vasc. Med. 2004, 9, 205–213. [Google Scholar] [CrossRef]
- Manero, F.; Ljubic-Thibal, V.; Moulin, M.; Goutagny, N.; Yvin, J. C.; Arrigo, A. P. Stimulation of Fas agonistic antibody-mediated apoptosis by heparin-like agents suppresses Hsp27 but not Bcl-2 protective activity. Cell Stress Chaperones 2004, 9, 150–166. [Google Scholar] [CrossRef]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef]
- Parish, C. R.; Coombe, D. R.; Jakobsen, K. B.; Bennett, F. A.; Underwood, P. A. Evidence that sulphated polysaccharides inhibit tumour metastasis by blocking tumour-cell-derived heparanases. Int. J. Cancer 1987, 40, 511–518. [Google Scholar] [CrossRef]
- Isnard, N.; Robert, L.; Renard, G. Effect of sulfated GAGs on the expression and activation of MMP-2 and MMP-9 in corneal and dermal explant cultures. Cell Biol. Int. 2003, 27, 779–784. [Google Scholar] [CrossRef]
- Aggarwal, B. B.; Shishodia, S.; Sandur, S. K.; Pandey, M. K.; Sethi, G. Inflammation and cancer: how hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R. K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef]
- Rusnati, M.; Tanghetti, E.; Urbinati, C.; Tulipano, G.; Marchesini, S.; Ziche, M.; Presta, M. Interaction of fibroblast growth factor-2 (FGF-2) with free gangliosides: biochemical characterization and biological consequences in endothelial cell cultures. Mol. Biol. Cell 1999, 10, 313–327. [Google Scholar] [CrossRef]
- Mukherjee, P.; Faber, A. C.; Shelton, L. M.; Baek, R. C.; Chiles, T. C.; Seyfried, T. N. Ganglioside GM3 suppresses the pro-angiogenic effects of vascular endothelial growth factor and ganglioside GD1A. J. Lipid Res. 2008, in press. [Google Scholar]
- Takahashi, H.; Ebihara, S.; Okazaki, T.; Asada, M.; Sasaki, H.; Yamaya, M. A comparison of the effects of unfractionated heparin, dalteparin and danaparoid on vascular endothelial growth factor-induced tumour angiogenesis and heparanase activity. Br. J. Pharmacol. 2005, 146, 333–343. [Google Scholar] [CrossRef]
- Ashikari-Hada, S.; Habuchi, H.; Kariya, Y.; Kimata, K. Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins. J. Biol. Chem. 2005, 280, 31508–31515. [Google Scholar] [CrossRef]
- Pisano, C.; Aulicino, C.; Vesci, L.; Casu, B.; Naggi, A.; Torri, G.; Ribatti, D.; Belleri, M.; Rusnati, M.; Presta, M. Undersulfated, low-molecular-weight glycol-split heparin as an antiangiogenic VEGF antagonist. Glycobiology 2005, 15, 1C–6C. [Google Scholar]
- Norrby, K. 2.5 kDa and 5.0 kDa heparin fragments specifically inhibit microvessel sprouting and network formation in VEGF165-mediated mammalian angiogenesis. Int. J. Exp. Pathol. 2000, 81, 191–198. [Google Scholar] [CrossRef]
- Nikitovic, D.; Assouti, M.; Sifaki, M.; Katonis, P.; Krasagakis, K.; Karamanos, N. K.; Tzanakakis, G. N. Chondroitin sulfate and heparan sulfate-containing proteoglycans are both partners and targets of basic fibroblast growth factor-mediated proliferation in human metastatic melanoma cell lines. Int. J. Biochem. Cell Biol. 2008, 40, 72–83. [Google Scholar] [CrossRef]
- Casu, B.; Guerrini, M.; Naggi, A.; Perez, M.; Torri, G.; Ribatti, D.; Carminati, P.; Giannini, G.; Penco, S.; Pisano, C.; Belleri, M.; Rusnati, M.; Presta, M. Short heparin sequences spaced by glycol-split uronate residues are antagonists of fibroblast growth factor 2 and angiogenesis inhibitors. Biochemistry 2002, 41, 10519–10528. [Google Scholar] [CrossRef]
- Casu, B.; Guerrini, M.; Guglieri, S.; Naggi, A.; Perez, M.; Torri, G.; Cassinelli, G.; Ribatti, D.; Carminati, P.; Giannini, G.; Penco, S.; Pisano, C.; Belleri, M.; Rusnati, M.; Presta, M. Undersulfated and glycol-split heparins endowed with antiangiogenic activity. J. Med. Chem. 2004, 47, 838–848. [Google Scholar] [CrossRef]
- Abe, W.; Ikejima, K.; Lang, T.; Okumura, K.; Enomoto, N.; Kitamura, T.; Takei, Y.; Sato, N. Low molecular weight heparin prevents hepatic fibrogenesis caused by carbon tetrachloride in the rat. J. Hepatol. 2007, 46, 286–294. [Google Scholar] [CrossRef]
- Cheng, J. J.; Huang, N. K.; Chang, T. T.; Wang, D. L.; Lu, M. K. Study for anti-angiogenic activities of polysaccharides isolated from Antrodia cinnamomea in endothelial cells. Life Sci. 2005, 76, 3029–3042. [Google Scholar] [CrossRef]
- Sakiyama, R.; Fukuta, K.; Matsumoto, K.; Furukawa, M.; Takahashi, Y.; Nakamura, T. Stimulation of hepatocyte growth factor production by heparin-derived oligosaccharides. J. Biochem. (Tokyo) 2007, 141, 653–660. [Google Scholar] [CrossRef]
- Rusnati, M.; Coltrini, D.; Oreste, P.; Zoppetti, G.; Albini, A.; Noonan, D.; d'Adda di Fagagna, F.; Giacca, M.; Presta, M. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. J. Biol. Chem. 1997, 272, 11313–11320. [Google Scholar]
- Albini, A.; Benelli, R.; Presta, M.; Rusnati, M.; Ziche, M.; Rubartelli, A.; Paglialunga, G.; Bussolino, F.; Noonan, D. HIV-tat protein is a heparin-binding angiogenic growth factor. Oncogene 1996, 12, 289–297. [Google Scholar]
- Zou, P.; Zou, K.; Muramatsu, H.; Ichihara-Tanaka, K.; Habuchi, O.; Ohtake, S.; Ikematsu, S.; Sakuma, S.; Muramatsu, T. Glycosaminoglycan structures required for strong binding to midkine, a heparin-binding growth factor. Glycobiology 2003, 13, 35–42. [Google Scholar]
- Soncin, F.; Strydom, D. J.; Shapiro, R. Interaction of heparin with human angiogenin. J. Biol. Chem. 1997, 272, 9818–9824. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y. J.; Yu, Q. Angiopoietin-3 is tethered on the cell surface via heparan sulfate proteoglycans. J. Biol. Chem. 2004, 279, 41179–41188. [Google Scholar] [CrossRef]
- Deepa, S. S.; Umehara, Y.; Higashiyama, S.; Itoh, N.; Sugahara, K. Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors. Implications as a physiological binding partner in the brain and other tissues. J. Biol. Chem. 2002, 277, 43707–43716. [Google Scholar]
- Fthenou, E.; Zafiropoulos, A.; Katonis, P.; Tsatsakis, A.; Karamanos, N. K.; Tzanakakis, G. N. Chondroitin sulfate prevents platelet derived growth factor-mediated phosphorylation of PDGF-Rbeta in normal human fibroblasts severely impairing mitogenic responses. J. Cell Biochem. 2008, 103, 1866–1876. [Google Scholar] [CrossRef]
- Kawada, A.; Hiura, N.; Tajima, S.; Takahara, H. Alginate oligosaccharides stimulate VEGF-mediated growth and migration of human endothelial cells. Arch. Dermatol. Res. 1999, 291, 542–547. [Google Scholar]
- Hoffman, R.; Burns, W. W., 3rd; Paper, D. H. Selective inhibition of cell proliferation and DNA synthesis by the polysulphated carbohydrate l-carrageenan. Cancer Chemother. Pharmacol. 1995, 36, 325–334. [Google Scholar] [CrossRef]
- Hoffman, R.; Sykes, D. Inhibition of binding of basic fibroblast growth factor to low and high affinity receptors by carrageenans. Biochem. Pharmacol. 1993, 45, 2348–2351. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Giraux, J. L.; Matou, S.; Bros, A.; Tapon-Bretaudiere, J.; Letourneur, D.; Fischer, A. M. Modulation of human endothelial cell proliferation and migration by fucoidan and heparin. Eur. J. Cell Biol. 1998, 77, 352–359. [Google Scholar]
- Dias, P. F.; Siqueira, J. M., Jr.; Vendruscolo, L. F.; de Jesus Neiva, T.; Gagliardi, A. R.; Maraschin, M.; Ribeiro-do-Valle, R. M. Antiangiogenic and antitumoral properties of a polysaccharide isolated from the seaweed Sargassum stenophyllum. Cancer Chemother. Pharmacol. 2005, 56, 436–446. [Google Scholar] [CrossRef]
- Catlow, K. R.; Deakin, J. A.; Wei, Z.; Delehedde, M.; Fernig, D. G.; Gherardi, E.; Gallagher, J. T.; Pavao, M. S.; Lyon, M. Interactions of hepatocyte growth factor / scatter factor with various glycosaminoglycans reveal an important interplay between the presence of iduronate and sulfate density. J. Biol. Chem. 2008, 283, 5235–5248. [Google Scholar]
- Miao, H. Q.; Ishai-Michaeli, R.; Peretz, T.; Vlodavsky, I. Laminarin sulfate mimics the effects of heparin on smooth muscle cell proliferation and basic fibroblast growth factor-receptor binding and mitogenic activity. J. Cell. Physiol. 1995, 164, 482–490. [Google Scholar] [CrossRef]
- Wang, L.; Geng, M.; Li, J.; Guan, H.; Ding, J. Studies of marine sulfated polymannuroguluronate on endothelial cell proliferation and endothelial immunity and related mechanisms. J. Pharmacol. Sci. 2003, 92, 367–373. [Google Scholar] [CrossRef]
- Lu, C. X.; Li, J.; Sun, Y. X.; Qi, X.; Wang, Q. J.; Xin, X. L.; Geng, M. Y. Sulfated polymannuroguluronate, a novel anti-AIDS drug candidate, inhibits HIV-1 Tat-induced angiogenesis in Kaposi's sarcoma cells. Biochem. Pharmacol. 2007, 74, 1330–1339. [Google Scholar] [CrossRef]
- Kobayashi, T.; Honke, K.; Miyazaki, T.; Matsumoto, K.; Nakamura, T.; Ishizuka, I.; Makita, A. Hepatocyte growth factor specifically binds to sulfoglycolipids. J. Biol. Chem. 1994, 269, 9817–9821. [Google Scholar]
- Kurosawa, N.; Kadomatsu, K.; Ikematsu, S.; Sakuma, S.; Kimura, T.; Muramatsu, T. Midkine binds specifically to sulfatide the role of sulfatide in cell attachment to midkine-coated surfaces. Eur. J. Biochem. 2000, 267, 344–351. [Google Scholar] [CrossRef]
- Letourneur, D.; Machy, D.; Pelle, A.; Marcon-Bachari, E.; D'Angelo, G.; Vogel, M.; Chaubet, F.; Michel, J. B. Heparin and non-heparin-like dextrans differentially modulate endothelial cell proliferation: in vitro evaluation with soluble and crosslinked polysaccharide matrices. J. Biomed. Mater. Res. 2002, 60, 94–100. [Google Scholar] [CrossRef]
- Hamma-Kourbali, Y.; Vassy, R.; Starzec, A.; Le Meuth-Metzinger, V.; Oudar, O.; Bagheri-Yarmand, R.; Perret, G.; Crepin, M. Vascular endothelial growth factor 165 (VEGF(165)) activities are inhibited by carboxymethyl benzylamide dextran that competes for heparin binding to VEGF(165) and VEGF(165).KDR Complexes. J. Biol. Chem. 2001, 276, 39748–39754. [Google Scholar]
- Borawski, J.; Dubowski, M.; Pawlak, K.; Mysliwiec, M. Sulodexide induces hepatocyte growth factor release in humans. Eur. J. Pharmacol. 2007, 558, 167–171. [Google Scholar] [CrossRef]
- Matou, S.; Colliec-Jouault, S.; Galy-Fauroux, I.; Ratiskol, J.; Sinquin, C.; Guezennec, J.; Fischer, A. M.; Helley, D. Effect of an oversulfated exopolysaccharide on angiogenesis induced by fibroblast growth factor-2 or vascular endothelial growth factor in vitro. Biochem. Pharmacol. 2005, 69, 751–759. [Google Scholar] [CrossRef]
- Ishihara, M.; Ono, K.; Ishikawa, K.; Hattori, H.; Saito, Y.; Yura, H.; Akaike, T.; Ozeki, Y.; Tanaka, S.; Mochizuki, H.; Kurita, A. Enhanced ability of heparin-carrying polystyrene (HCPS) to bind to heparin-binding growth factors and to inhibit growth factor-induced endothelial cell growth. J. Biochem. (Tokyo) 2000, 127, 797–803. [Google Scholar] [CrossRef]
- de Paz, J. L.; Noti, C.; Bohm, F.; Werner, S.; Seeberger, P. H. Potentiation of fibroblast growth factor activity by synthetic heparin oligosaccharide glycodendrimers. Chem. Biol. 2007, 14, 879–887. [Google Scholar] [CrossRef]
- Kim, S. H.; Kiick, K. L. Heparin-mimetic sulfated peptides with modulated affinities for heparin-binding peptides and growth factors. Peptides 2007, 28, 2125–2136. [Google Scholar] [CrossRef]
- Benelli, U.; Bocci, G.; Danesi, R.; Lepri, A.; Bernardini, N.; Bianchi, F.; Lupetti, M.; Dolfi, A.; Campagni, A.; Agen, C.; Nardi, M.; Del Tacca, M. The heparan sulfate suleparoide inhibits rat corneal angiogenesis and in vitro neovascularization. Exp. Eye Res. 1998, 67, 133–142. [Google Scholar] [CrossRef]
- Leali, D.; Belleri, M.; Urbinati, C.; Coltrini, D.; Oreste, P.; Zoppetti, G.; Ribatti, D.; Rusnati, M.; Presta, M. Fibroblast growth factor-2 antagonist activity and angiostatic capacity of sulfated Escherichia coli K5 polysaccharide derivatives. J. Biol. Chem. 2001, 276, 37900–37908. [Google Scholar]
- Presta, M.; Oreste, P.; Zoppetti, G.; Belleri, M.; Tanghetti, E.; Leali, D.; Urbinati, C.; Bugatti, A.; Ronca, R.; Nicoli, S.; Moroni, E.; Stabile, H.; Camozzi, M.; Hernandez, G. A.; Mitola, S.; Dell'Era, P.; Rusnati, M.; Ribatti, D. Antiangiogenic activity of semisynthetic biotechnological heparins: low-molecular-weight-sulfated Escherichia coli K5 polysaccharide derivatives as fibroblast growth factor antagonists. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 71–76. [Google Scholar]
- Urbinati, C.; Bugatti, A.; Oreste, P.; Zoppetti, G.; Waltenberger, J.; Mitola, S.; Ribatti, D.; Presta, M.; Rusnati, M. Chemically sulfated Escherichia coli K5 polysaccharide derivatives as extracellular HIV-1 Tat protein antagonists. FEBS Lett. 2004, 568, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Joyce, J. A.; Freeman, C.; Meyer-Morse, N.; Parish, C. R.; Hanahan, D. A functional heparan sulfate mimetic implicates both heparanase and heparan sulfate in tumor angiogenesis and invasion in a mouse model of multistage cancer. Oncogene 2005, 24, 4037–4051. [Google Scholar] [CrossRef]
- Ferro, V.; Dredge, K.; Liu, L.; Hammond, E.; Bytheway, I.; Li, C.; Johnstone, K.; Karoli, T.; Davis, K.; Copeman, E.; Gautam, A. PI-88 and novel heparan sulfate mimetics inhibit angiogenesis. Semin. Thromb. Hemost. 2007, 33, 557–568. [Google Scholar] [CrossRef]
- Rouet, V.; Hamma-Kourbali, Y.; Petit, E.; Panagopoulou, P.; Katsoris, P.; Barritault, D.; Caruelle, J. P.; Courty, J. A synthetic glycosaminoglycan mimetic binds vascular endothelial growth factor and modulates angiogenesis. J. Biol. Chem. 2005, 280, 32792–32800. [Google Scholar] [CrossRef]
- Rouet, V.; Meddahi-Pelle, A.; Miao, H. Q.; Vlodavsky, I.; Caruelle, J. P.; Barritault, D. Heparin-like synthetic polymers, named RGTAs, mimic biological effects of heparin in vitro. J. Biomed. Mater. Res. A 2006, 78, 792–797. [Google Scholar]
- Fannon, M.; Forsten-Williams, K.; Nugent, M. A.; Gregory, K. J.; Chu, C. L.; Goerges-Wildt, A. L.; Panigrahy, D.; Kaipainen, A.; Barnes, C.; Lapp, C.; Shing, Y. Sucrose octasulfate regulates fibroblast growth factor-2 binding, transport, and activity: Potential for regulation of tumor growth. J. Cell Physiol. 2008, 215, 434–441. [Google Scholar] [CrossRef]
- Kasbauer, C. W.; Paper, D. H.; Franz, G. Sulfated beta-(1-->4)-galacto-oligosaccharides and their effect on angiogenesis. Carbohydr. Res. 2001, 330, 427–430. [Google Scholar] [CrossRef]
- Zugmaier, G.; Lippman, M. E.; Wellstein, A. Inhibition by pentosan polysulfate (PPS) of heparin-binding growth factors released from tumor cells and blockage by PPS of tumor growth in animals. J. Natl. Cancer Inst. 1992, 84, 1716–1724. [Google Scholar] [CrossRef]
- Rusnati, M.; Urbinati, C.; Caputo, A.; Possati, L.; Lortat-Jacob, H.; Giacca, M.; Ribatti, D.; Presta, M. Pentosan polysulfate as an inhibitor of extracellular HIV-1 Tat. J. Biol. Chem. 2001, 276, 22420–22425. [Google Scholar]
- Sakairi, N.; Kuzuhara, H.; Okamoto, T.; Yajima, M. Synthesis and biological evaluation of 2-amino-2-deoxy- and 6-amino-6-deoxy-cyclomaltoheptaose polysulfates as synergists for angiogenesis inhibition. Bioorg. Med. Chem. 1996, 4, 2187–2192. [Google Scholar] [CrossRef]
- Watson, K.; Gooderham, N. J.; Davies, D. S.; Edwards, R. J. Interaction of the transactivating protein HIV-1 tat with sulphated polysaccharides. Biochem. Pharmacol. 1999, 57, 775–783. [Google Scholar] [CrossRef]
- Waltenberger, J.; Mayr, U.; Frank, H.; Hombach, V. Suramin is a potent inhibitor of vascular endothelial growth factor. A contribution to the molecular basis of its antiangiogenic action. J. Mol. Cell Cardiol. 1996, 28, 1523–1529. [Google Scholar] [CrossRef]
- Schilling-Schon, A.; Pleyer, U.; Hartmann, C.; Rieck, P. W. The role of endogenous growth factors to support corneal endothelial migration after wounding in vitro. Exp. Eye Res. 2000, 71, 583–589. [Google Scholar] [CrossRef]
- Rusnati, M.; Dell'Era, P.; Urbinati, C.; Tanghetti, E.; Massardi, M. L.; Nagamine, Y.; Monti, E.; Presta, M. A distinct basic fibroblast growth factor (FGF-2)/FGF receptor interaction distinguishes urokinase-type plasminogen activator induction from mitogenicity in endothelial cells. Mol. Biol. Cell 1996, 7, 369–381. [Google Scholar] [CrossRef]
- Sola, F.; Farao, M.; Ciomei, M.; Pastori, A.; Mongelli, N.; Grandi, M. FCE 27266, a sulfonic distamycin derivative, inhibits experimental and spontaneous lung and liver metastasis. Invasion Metastasis 1995, 15, 222–231. [Google Scholar]
- Sola, F.; Gualandris, A.; Belleri, M.; Giuliani, R.; Coltrini, D.; Bastaki, M.; Molinari-Tosatti, M. P.; Bonardi, F.; Vecchi, A.; Fioretti, F.; Giavazzi, R.; Ciomei, M.; Grandi, M.; Mantovani, A.; Presta, M. Endothelial cells overexpressing basic fibroblast growth factor (FGF-2) induce vascular tumors in immunodeficient mice. Angiogenesis 1997, 1, 102–116. [Google Scholar] [CrossRef]
- Corallini, A.; Betti, M.; Rusnati, M.; Campioni, D.; Ciomei, M.; Sola, F.; Calza, N.; Zauli, G.; Presta, M.; Barbanti-Brodano, G.; Caputo, A. Characterization of the effects of two polysulfonated distamycin A derivatives, PNU145156E and PNU153429, on HIV type 1 Tat protein. AIDS Res. Hum. Retroviruses 1998, 14, 1561–1571. [Google Scholar]
- Liekens, S.; Leali, D.; Neyts, J.; Esnouf, R.; Rusnati, M.; Dell'Era, P.; Maudgal, P. C.; De Clercq, E.; Presta, M. Modulation of fibroblast growth factor-2 receptor binding, signaling, and mitogenic activity by heparin-mimicking polysulfonated compounds. Mol. Pharmacol. 1999, 56, 204–213. [Google Scholar]
- Bugatti, A.; Urbinati, C.; Ravelli, C.; De Clercq, E.; Liekens, S.; Rusnati, M. Heparin-mimicking sulfonic acid polymers as multitarget inhibitors of human immunodeficiency virus type 1 Tat and gp120 proteins. Antimicrob. Agents Chemother. 2007, 51, 2337–2345. [Google Scholar] [CrossRef]
- Terabayashi, T.; Morita, M.; Ueno, M.; Nakamura, T.; Urashima, T. Inhibition of influenza-virus-induced cytopathy by sialylglycoconjugates. Carbohydr. Res. 2006, 341, 2246–2253. [Google Scholar] [CrossRef]
- Kreuger, J.; Spillmann, D.; Li, J. P.; Lindahl, U. Interactions between heparan sulfate and proteins: the concept of specificity. J. Cell Biol. 2006, 174, 323–327. [Google Scholar] [CrossRef]
- Baba, M.; Schols, D.; De Clercq, E.; Pauwels, R.; Nagy, M.; Gyorgyi-Edelenyi, J.; Low, M.; Gorog, S. Novel sulfated polymers as highly potent and selective inhibitors of human immunodeficiency virus replication and giant cell formation. Antimicrob. Agents Chemother. 1990, 34, 134–138. [Google Scholar] [CrossRef]
- Schols, D.; Pauwels, R.; Desmyter, J.; De Clercq, E. Dextran sulfate and other polyanionic anti-HIV compounds specifically interact with the viral gp120 glycoprotein expressed by T-cells persistently infected with HIV-1. Virology 1990, 175, 556–561. [Google Scholar] [CrossRef]
- Baba, M.; Schols, D.; Pauwels, R.; Nakashima, H.; De Clercq, E. Sulfated polysaccharides as potent inhibitors of HIV-induced syncytium formation: a new strategy towards AIDS chemotherapy. J. Acquir. Immune Defic. Syndr. 1990, 3, 493–499. [Google Scholar]
- Mahoney, C. W.; Azzi, A.; Huang, K. P. Effects of suramin, an anti-human immunodeficiency virus reverse transcriptase agent, on protein kinase C. Differential activation and inhibition of protein kinase C isozymes. J. Biol. Chem. 1990, 265, 5424–5428. [Google Scholar]
- Yahi, N.; Sabatier, J. M.; Nickel, P.; Mabrouk, K.; Gonzalez-Scarano, F.; Fantini, J. Suramin inhibits binding of the V3 region of HIV-1 envelope glycoprotein gp120 to galactosylceramide, the receptor for HIV-1 gp120 on human colon epithelial cells. J. Biol. Chem. 1994, 269, 24349–24353. [Google Scholar]
- Witvrouw, M.; Fikkert, V.; Pluymers, W.; Matthews, B.; Mardel, K.; Schols, D.; Raff, J.; Debyser, Z.; De Clercq, E.; Holan, G.; Pannecouque, C. Polyanionic (i.e., polysulfonate) dendrimers can inhibit the replication of human immunodeficiency virus by interfering with both virus adsorption and later steps (reverse transcriptase/integrase) in the virus replicative cycle. Mol Pharmacol. 2000, 58, 1100–1108. [Google Scholar]
- Tan, G. T.; Wickramasinghe, A.; Verma, S.; Hughes, S. H.; Pezzuto, J. M.; Baba, M.; Mohan, P. Sulfonic acid polymers are potent inhibitors of HIV-1 induced cytopathogenicity and the reverse transcriptases of both HIV-1 and HIV-2. Biochim. Biophys. Acta 1993, 1181, 183–188. [Google Scholar]
- Noonan, D.; Albini, A. From the outside in: extracellular activities of HIV Tat. Adv. Pharmacol. 2000, 48, 229–250. [Google Scholar] [CrossRef]
- Eggert, A.; Ikegaki, N.; Kwiatkowski, J.; Zhao, H.; Brodeur, G. M.; Himelstein, B. P. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin. Cancer Res. 2000, 6, 1900–1908. [Google Scholar]
- Hammache, D.; Pieroni, G.; Yahi, N.; Delezay, O.; Koch, N.; Lafont, H.; Tamalet, C.; Fantini, J. Specific interaction of HIV-1 and HIV-2 surface envelope glycoproteins with monolayers of galactosylceramide and ganglioside GM3. J. Biol. Chem. 1998, 273, 7967–7971. [Google Scholar]
- Brown, G.; Rixon, H. W.; Sugrue, R. J. Respiratory syncytial virus assembly occurs in GM1-rich regions of the host-cell membrane and alters the cellular distribution of tyrosine phosphorylated caveolin-1. J. Gen. Virol. 2002, 83, 1841–1850. [Google Scholar]
- Low, J. A.; Magnuson, B.; Tsai, B.; Imperiale, M. J. Identification of gangliosides CD1b and GT1b as receptors for BK virus. J. Virol. 2006, 80, 1361–1366. [Google Scholar] [CrossRef]
- Seeberger, P. H. Automated carbohydrate synthesis to drive chemical glycomics. Chem. Commun. (Camb) 2003, 1115–1121. [Google Scholar] [CrossRef]
- Feizi, T.; Fazio, F.; Chai, W.; Wong, C. H. Carbohydrate microarrays - a new set of technologies at the frontiers of glycomics. Curr. Opin. Struct. Biol. 2003, 13, 637–645. [Google Scholar] [CrossRef]
- Ashikari-Hada, S.; Habuchi, H.; Kariya, Y.; Itoh, N.; Reddi, A. H.; Kimata, K. Characterization of growth factor-binding structures in heparin/heparan sulfate using an octasaccharide library. J. Biol. Chem. 2004, 279, 12346–12354. [Google Scholar]
- Freeman, C.; Liu, L.; Banwell, M. G.; Brown, K. J.; Bezos, A.; Ferro, V.; Parish, C. R. Use of sulfated linked cyclitols as heparan sulfate mimetics to probe the heparin/heparan sulfate binding specificity of proteins. J. Biol. Chem. 2005, 280, 8842–8849. [Google Scholar]
- Rusnati, M.; Oreste, P.; Zoppetti, G.; Presta, M. Biotechnological engineering of heparin/heparan sulphate: a novel area of multi-target drug discovery. Curr. Pharm. Des. 2005, 11, 2489–2499. [Google Scholar] [CrossRef]
- Lubineau, A.; Lortat-Jacob, H.; Gavard, O.; Sarrazin, S.; Bonnaffe, D. Synthesis of tailor-made glycoconjugate mimetics of heparan sulfate that bind IFN-gamma in the nanomolar range. Chemistry 2004, 10, 4265–4282. [Google Scholar] [CrossRef]
- Krieger, E.; Geretti, E.; Brandner, B.; Goger, B.; Wells, T. N.; Kungl, A. J. A structural and dynamic model for the interaction of interleukin-8 and glycosaminoglycans: support from isothermal fluorescence titrations. Proteins 2004, 54, 768–775. [Google Scholar] [CrossRef]
- Raj, P. A.; Marcus, E.; Rein, R. Conformational requirements of suramin to target angiogenic growth factors. Angiogenesis 1998, 2, 183–199. [Google Scholar] [CrossRef]
- Sample Availability: contact the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Urbinati, C.; Chiodelli, P.; Rusnati, M. Polyanionic Drugs and Viral Oncogenesis: a Novel Approach to Control Infection, Tumor-associated Inflammation and Angiogenesis. Molecules 2008, 13, 2758-2785. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules13112758
Urbinati C, Chiodelli P, Rusnati M. Polyanionic Drugs and Viral Oncogenesis: a Novel Approach to Control Infection, Tumor-associated Inflammation and Angiogenesis. Molecules. 2008; 13(11):2758-2785. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules13112758
Chicago/Turabian StyleUrbinati, Chiara, Paola Chiodelli, and Marco Rusnati. 2008. "Polyanionic Drugs and Viral Oncogenesis: a Novel Approach to Control Infection, Tumor-associated Inflammation and Angiogenesis" Molecules 13, no. 11: 2758-2785. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules13112758