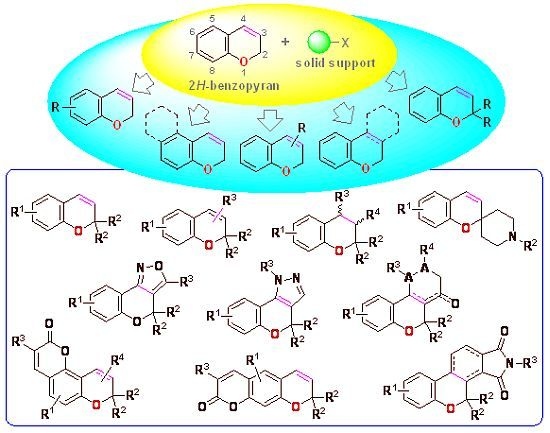
Solid-Phase Parallel Synthesis of Drug-Like Artificial 2H-Benzopyran Libraries
Abstract
:1. Introduction


2. Solid-Phase Synthesis of Substituted Benzopyran Compounds
2.1. Solid-Phase Synthesis of Monosubstituted Benzopyran Compounds
2.1.1. Solid-Phase Synthesis of 3-Substituted Benzopyran Compounds


2.1.2. Solid-Phase Synthesis of 4-Substituted Benzopyran Compounds



2.1.3. Solid-Phase Synthesis of 6-Substituted Benzopyran Compounds

2.1.4. Solid-Phase Synthesis of 8-Substituted Benzopyran Compounds

2.2. Solid-Phase Synthesis of Disubstituted Benzopyran Compounds
2.2.1. Solid-phase Synthesis of 2,3-Disubstituted Benzopyran Compounds
2.2.2. Solid-Phase Synthesis of 2,6-Disubstituted Benzopyran Compounds



2.3. Solid-Phase Synthesis of Trisubstituted Benzopyran Compounds
2.3.1. Solid-Phase Synthesis of 2,4,6-Trisubstituted Benzopyran Compounds

2.3.2. Solid-Phase Synthesis of 3,4,6-Trisubstituted Benzopyran Compounds

3. Solid-Phase Synthesis of Additional Cycle-Fused Benzopyran Compounds
3.1. Solid-Phase Synthesis of Tricyclic Benzopyran Compounds
3.1.1. Solid-Phase Synthesis of Isoxazole-Fused Benzopyran Compounds

3.1.2. Solid-Phase Synthesis of Pyrazole-Fused Benzopyran Compounds

3.1.3. Solid-Phase Synthesis of Six-Membered Ring-Fused Benzopyran Compounds


3.2. Solid-Phase Synthesis of Polycyclic Benzopyran Compounds

4. Solid-Phase Synthesis of Miscellaneous Benzopyran Compounds


5. Summary
Acknowledgments
References and Notes
- Dolle, R.E.; le Bourdonnec, B.; Worm, K.; Morales, G.A.; Thomas, C.J.; Zhang, W. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2009. J. Comb. Chem. 2010, 12, 765–806. [Google Scholar] [CrossRef]
- Dolle, R.E.; le Bourdonnec, B.; Goodman, A.J.; Morales, G.A.; Thomas, C.J.; Zhang, W. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2008. J. Comb. Chem. 2009, 11, 739–790. [Google Scholar] [CrossRef]
- Dolle, R.E.; le Bourdonnec, B.; Goodman, A.J.; Morales, G.A.; Thomas, C.J.; Zhang, W. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2007. J. Comb. Chem. 2008, 10, 753–800. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Williams, L.; Bridges, T.M.; Daniels, R.N.; Weaver, D.; Lindsley, C.W. Application of combinatorial chemistry science on modern drug discovery. J. Comb. Chem. 2008, 10, 345–354. [Google Scholar] [CrossRef]
- Nicolaou, K.C., Hanko, R., Hartwig, W.; Handbook of Combinatorial Chemistry: Drugs, Catalysts (Ed.) Wiley-VCH: Weinheim, Germany, 2002.
- Dandapani, S.; Marcaurelle, L.A. Accessing new chemical space for “undruggable” targets. Nat. Chem. Biol. 2010, 6, 861–863. [Google Scholar]
- Drewry, D.H.; Macarron, R. Enhancements of screening collections to address areas of unmet medical need: an industry perspective. Curr. Opin. Chem. Biol. 2010, 14, 289–298. [Google Scholar] [CrossRef]
- Bleicher, K.H.; Böhm, H.-J.; Müller, K.; Alanine, A.I. Hit and lead generation: Beyond high-throughput screening. Nat. Rev. Drug Discov. 2003, 2, 369–378. [Google Scholar] [CrossRef]
- Horton, D.A.; Bourne, G.T.; Smyth, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef]
- Krchnňák, V.; Holladay, M.W. Solid phase heterocylic chemistry. Chem. Rev. 2002, 102, 61–91. [Google Scholar]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef]
- Bannwarth, W., Hinzen, B., 2nd; Combinatorial Chemistry: From Theory to, Application (Ed.) Wiley-VCH: Weinheim, Germany, 2006.
- Gil, C.; Bräs, S. Solid-phase synthesis of biologically active benzoannelated nitrogen heterocycles: An update. J. Comb. Chem. 2009, 9, 175–197. [Google Scholar]
- Ziegert, R.E.; Toräng, J.; Knepper, K.; Bräse, S. The recent impact of solid-phase synthesis on medicinally relevant benzoannelated oxygen heterocycles. J. Comb. Chem. 2005, 7, 147–169. [Google Scholar] [CrossRef]
- Franzén, R.G. Recent advances in the preparation of heterocycles on solid support: A review of the literature. J. Comb. Chem. 2000, 2, 195–214. [Google Scholar] [CrossRef]
- Fang, N.; Casida, J.E. Anticancer action of cubé insecticide: Correlation for rotenoid constituents between inhibition of NADH: Ubiquinone oxidoreductase and induced ornithine decarboxylase activities. Proc. Natl. Acad. Sci. USA 1998, 95, 3380–3384. [Google Scholar] [CrossRef]
- Fang, N.; Casida, J.E. New Bioactive Flavonoids and Stilbenes in Cubé Resin Insecticide. J. Nat. Prod. 1999, 62, 205–210. [Google Scholar] [CrossRef]
- Kaouadji, M.; Agban, A.; Mariotte, A.M.; Tissut, M. Lonchocarpene, a Stilbene, and Lonchocarpusone, an Isoflavone: Two new pyranopolyphenols from lonchocarpus nicou roots. J. Nat. Prod. 1986, 49, 281–285. [Google Scholar] [CrossRef]
- Takasugi, M.; Nagao, S.; Ueno, S.; Masamune, T.; Shirata, A.; Takahashi, K. Studies on phytoalexins of the Moraceae. 2. Moracin C and D, new phytoalexins from diseased mulberry. Chem. Lett. 1978, 1239–1240. [Google Scholar]
- Shirata, A.; Takahashi, K.; Takasugi, M.; Nagao, S.; Ishikawa, S.; Ueno, S.; Munoz, L.; Masamune, T. Antimircrobial spectra of the compounds from mulberry. Sanshi Shikenjo Hokoku 1983, 28, 793–806. [Google Scholar]
- Tyrrell, E.; Tesfa, K.H.; Greenwood, I.; Mann, A. The synthesis and biological evaluation of a range of novel functionalised benzopyrans as potential potassium channel activators. Bioorg. Med. Chem. Lett. 2008, 18, 1237–1240. [Google Scholar] [CrossRef]
- Drizin, I.; Holladay, M.W.; Lin, Y.; Zhang, H.Q.; Gopalakrishnan, S.; Gopalakrishnan, M.; Whiteaker, K.L.; Buckner, S.A.; Sullivan, J.P.; Carroll, W.A. Structure-activity studies for a novel series of tricyclic dihydropyrimidines as KATP channel openers (KCOs). Bioorg. Med. Chem. Lett. 2002, 12, 1481–1484. [Google Scholar]
- Chiu, H.-I.; Lin, Y.-C.; Cheng, C.-Y.; Tsai, M.-C.; Yu, H.-C. N-Acyl-1,2,3,4a,5, 10b-hexahydro-[1]benzopyrano-[3,4-b][1,4]oxazine -9-carbonitriles as bladder-selective potassium channel openers. Bioorg. Med. Chem. 2001, 9, 383–393. [Google Scholar] [CrossRef]
- Andersson, K.-E. Clinical pharmacology of potassium channel openers. Pharmacol. Toxicol. 1992, 70, 244–254. [Google Scholar] [CrossRef]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef]
- Carter, J.S.; Obukowicz, M.G.; Devedas, B.; Talley, J.J.; Brown, D.L.; Graneto, M.J.; Bertenshaw, S.R.; Rogier, D.J.; Srinivasan, R.; Hanau, C.E.; et al. Substituted Benzopyran Derivatives for the Treatment of Inflammation. WO 9847890 A1, 1998. [Google Scholar]
- Stuart, E.C.; Scandlyn, M.J.; Rosengren, R.J. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sci. 2006, 79, 2329–2336. [Google Scholar] [CrossRef]
- Fujimura, Y.; Yamada, K.; Tachibana, H. A lipid raft-associated 67kDa laminin receptor mediates suppressive effect of epigallocatechin-3-O-gallate on FceRI expression. Biochem. Biophys. Res. Commun. 2005, 336, 674–681. [Google Scholar] [CrossRef]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar]
- Gong, Y.-D.; Yoo, S.-E.; Cheon, H.G.; Cho, Y.S.; Seo, J.-S.; Hwang, J.Y.; Park, J.Y. 2,2'-Disubstituted-3,4-dehydro-7,8-disubstituted-6-alkylamino benzopyran derivatives as 5-lipoxgenase inhibitor. Korea Patent 10–0602191, 2006. [Google Scholar]
- Gong, Y.-D.; Yoo, S.-E.; Cheon, H.G.; Cho, Y.S.; Seo, J.-S.; Hwang, J.Y.; Park, J.Y. Preparation of 6-Alkylamino-2,2'-disubstituted-7,8-disubstituted-2H-1-benzopyran Derivatives as 5-Lipoxgenase Inhibitor. US 736 2005. [Google Scholar]
- Gunatilaka, A.A.L.; Kingston, D.G.I.; Wijeratne, E.M.K.; Bandara, B.M.R.; Hofmann, G.A.; Johnson, R.K. Biological activity of some coumarins from sri lankan rutaceae. J. Nat. Prod. 1994, 57, 518–520. [Google Scholar] [CrossRef]
- Bandara, B.M.R.; Gunatilaka, A.A.L.; Wijeratne, E.M.K.; MacLeod, J.K. Acridone alkaloids and coumarins from Pleiospermium alatum. Phytochemistry 1990, 29, 297–301. [Google Scholar]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.-L.; Mitaku, S.; Leonce, S.; Renard, P.; Pierre, A.; Atassi, G. Synthesis and cytotoxic activity of pyranocoumarins of the seselin and xanthyletin series. J. Nat. Prod. 1998, 61, 982–986. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Afaq, F.; Mukhtar, H. Cannabinoids for cancer treatment: Progress and promise. Cancer Res. 2008, 68, 339–342. [Google Scholar] [CrossRef]
- Ware, M.A.; Daeninck, P.; Maida, V. A review of nabilone in the treatment of chemotherapy-induced nausea and vomiting. Ther. Clin. Risk Manag. 2008, 4, 99–107. [Google Scholar]
- Pertwee, R.G. Cannabinoids and multiple sclerosis. Mol. Neurobiol. 2007, 36, 45–49. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 101–122. [Google Scholar] [CrossRef]
- Romero, J.; Lastres-Becker, L.; de Miguel, R.; Berrendero, F.; Ramos, J.A.; Fernandez-Ruiz, J. The endogenous cannabinoid system and the basal ganglia: biochemical, pharmacological, and therapeutic aspects. Pharmacol. Ther. 2002, 95, 137–152. [Google Scholar] [CrossRef]
- Lee, T.T.-Y.; Kashiwada, Y.; Huang, L.; Snider, J.; Cosentino, L.M.; Lee, K.H. Suksdorfin: An anti-HIV principle from Lomatium suksdorfii, its structure-activity correlation with related coumarins, and synergistic effects with anti-AIDS nucleosides. Bioorg. Med. Chem. 1994, 2, 1051–1056. [Google Scholar] [CrossRef]
- Willette, R.E.; Soine, T.O. Isolation, purification, and structure determination of pteryxin and suksdorfin. J. Pharm. Sci. 1962, 51, 149–156. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Eggleston, D.S.; Haltiwanger, R.C.; Tomcowicz, B.; Breen, A.; Johnson, R.K. Rigidone, a sesquiterpene o-quinone from the gorgonian Pseudopterogorgia rigida. J. Nat. Prod. 1997, 60, 306–308. [Google Scholar] [CrossRef]
- Zhou, Z.-Z.; Yang, G.-F. Insecticidal lead identification by screening benzopyrano[4,3-c]-pyrazol-3(2H)-ones library constructed from multiple-parallel synthesis under microwave irradiation. Bioorg. Med. Chem. 2006, 14, 8666–8674. [Google Scholar] [CrossRef]
- Yenesew, A.; Derese, S.; Midiwo, J.O.; Heydenreich, M.; Peter, M.G. Effect of rotenoids from the seeds of Millettia dura on larvae of Aedes aegypti. Pest. Manag. Sci. 2003, 59, 1159–1161. [Google Scholar] [CrossRef]
- Cabizza, M.; Angioni, A.; Melis, M.; Cabras, M.; Tuberoso, C.V.; Cabras, P. Rotenone and rotenoids in cubè resins, formulations, and residues on olives. J. Agric. Food Chem. 2004, 52, 288–293. [Google Scholar]
- Nussbaumer, P.; Lehr, P.; Billich, A. 2-Substituted 4-(Thio)chromenone 6-O-Sulfamates: Potent inhibitors of human steroid sulfatase. J. Med. Chem. 2002, 45, 4310–4320. [Google Scholar] [CrossRef]
- Joo, Y.H.; Kim, J.K.; Kang, S.-H.; Noh, M.-S.; Ha, J.-Y.; Choi, J.K.; Lim, K.M.; Lee, C.H.; Chung, S. 2,3-Diarylbenzopyran derivatives as a novel class of selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 413–417. [Google Scholar]
- Pal, M.; Subramanian, V.; Parasuraman, K.; Yeleswarapu, K.R. Palladium catalyzed reaction in aqueous DMF: Synthesis of 3-alkynyl substituted flavones in the presence of prolinol. Tetrahedron 2003, 59, 9563–9570. [Google Scholar]
- Maliar, T.; Jedinak, A.; Kadrabova, J.; Sturdik, E. Structural aspects of flavonoids as trypsin inhibitors. Eur. J. Med. Chem. 2004, 39, 241–248. [Google Scholar] [CrossRef]
- Zhu, M.; Lim, B.J.; Koh, M.; Park, S.B. Construction of polyheterocyclic benzopyran library with diverse core skeletons through diversity-oriented synthesis pathway: Part II. ACS Comb. Sci. 2012, 14, 124–134. [Google Scholar]
- Oh, S.; Jang, H.J.; Ko, S.K.; Ko, Y.; Park, S.B. Construction of a polyheterocyclic benzopyran library with diverse core skeletons through diversity-oriented synthesis pathway. J. Comb. Chem. 2010, 12, 548–558. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI-Catalyzed Alkyne-Azide “Click” Cycloadditions from a mechanistic and synthetic perspective. Eur. J. Org. Chem. 2006, 51–68. [Google Scholar]
- Pérez-Balderas, F.; Ortega-Muñoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; Calvo-Flores, F.G.; Calvo-Asín, J.A.; Isac-García, J.; Santoyo-González, F. Multivalent neoglycoconjugates by regiospecfic cycloaddition of alkynes and azides using organic-soluble copper catalysts. Org. Lett. 2003, 5, 1951–1954. [Google Scholar]
- Gong, Y.-D.; Yoo, S.-E. Solid-phase synthesis of benzopyran derivatives via highly efficient epoxidation using two-phase solvents. Bull. Korean Chem. Soc. 2001, 22, 941–942. [Google Scholar]
- Gong, Y.-D.; Seo, J.-S.; Chon, Y.S.; Hwang, J.Y.; Park, J.Y.; Yoo, S.-E. Construction of 6-amino-2,2-dimethyl-3,4,6-trisubstituted-2h-1-benzopyran library by solid phase synthesis. J. Comb. Chem. 2003, 5, 577–589. [Google Scholar] [CrossRef]
- Alvarez-Gutierrez, J.M.; Nefzi, A.; Houghten, R.A. Solid phase synthesis of 1-substituted pyroglutamates. Tetrahedron Lett. 2000, 41, 851–854. [Google Scholar] [CrossRef]
- Huang, W.; Scaborough, R.M. A new “Traceless” solid-phase synthesis strategy: Synthesis of a benzimidazole library. Tetrahedron Lett. 1999, 40, 2665–2668. [Google Scholar] [CrossRef]
- Xing, L.; Hamper, B.C.; Fletcher, T.R.; Wendling, J.M.; Carter, J.; Gierse, J.M.; Liao, S. Structure-based parallel medicinal chemistry approach to improve metabolic stability of benzopyran COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 993–996. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Tanaka, H.; Miyoshi, H.; Chuang, Y.-C.; Ando, Y.; Takahashi, T. Solid-phase synthesis of epigallocatechin gallate derivatives. Angew. Chem. Int. Ed. Engl. 2007, 46, 5934–5937. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamanouchi, M.; Miyoshi, H.; Hirotsu, K.; Tachibana, H.; Takahashi, T. Solid-phase synthesis of a combinatorial methylated (−)-epigallocatechin gallate library and the growth-inhibitory effects of these compounds on melanoma B16 cells. Chem. Asian J. 2010, 5, 2231–2248. [Google Scholar] [CrossRef]
- Micheli, F.; Degiorgis, F.; Feriani, A.; Paio, A.; Pozzan, A.; Zarantonello, P.; Seneci, P. A combinatorial approach to [1,5]benzothiazepine derivatives as potential antibacterial agents. J. Comb. Chem. 2001, 3, 224–228. [Google Scholar] [CrossRef]
- Tosaki, S.; Tsuji, R.; Ohshima, T.; Shibasaki, M. Dynamic ligand exchange of the lanthanide complex leading to structural and functional transformation: One-pot sequential catalytic asymmetric epoxidation-regioselective epoxide-opening process. J. Am. Chem. Soc. 2005, 127, 2147–2155. [Google Scholar]
- Hwang, J.Y.; Choi, H.-S.; Seo, J.-S.; La, H.J.; Kim, D.-S.; Jeon, H.S.; Jeon, M.-K.; Lee, D.-H.; Gong, Y.-D. Construction of a 2,6-difunctionalized 2-methyl-2H-1-benzopyran library by using a solid-phase synthesis protocol. J. Org. Chem. 2005, 70, 10151–10154. [Google Scholar]
- Fivush, A.M.; Wilson, T.M. AMEBA: An acid sensitive aldehyde resin for solid phase synthesis. Tetrahedron Lett. 1997, 38, 7151–7154. [Google Scholar] [CrossRef]
- Ouyang, X.; Tamayo, N.; Kiselyov, A.S. Solid support synthesis of 2-substituted dibenz[b,f]oxazepin-11(10H)-ones via SNAr methodology on AMEBA resin. Tetrahedron 1999, 55, 2827–2834. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Toader, D.; Watson, K.; Kiely, J.S. New synthesis of SASRINTM resin. Tetrahedron Lett. 1997, 38, 7849–7850. [Google Scholar]
- Yoo, S.-E.; Yi, K.Y.; Lee, S.; Suh, J.; Kim, N.; Lee, B.H.; Seo, H.W.; Kim, S.-O.; Lee, D.-H.; Lim, H.; Shin, H.S. A novel anti-ischemic ATP-sensitive potassium channel (KATP) opener without vasorelaxation: N-(6-Aminobenzopyranyl)-N'-benzyl-N''-cyanoguanidine analogue. J. Med. Chem. 2001, 44, 4207–4215. [Google Scholar] [CrossRef]
- Boojamra, C.G.; Burow, K.M.; Thompson, L.A.; Ellman, J.A. Solid-phase synthesis of 1,4-benzodiazepine-2,5-diones. Library preparation and demonstration of synthesis generality. J. Org. Chem. 1997, 62, 1240–1256. [Google Scholar] [CrossRef]
- Arya, P.; Wei, C.-Q.; Barnes, M.L.; Daroszewska, M. A solid phase library synthesis of hydroxyindoline-derived tricyclic derivatives by mitsunobu approach. J. Comb. Chem. 2004, 6, 65–72. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Choi, H.-S.; Seo, J.-S.; La, H.-J.; Yoo, S.-E.; Gong, Y.-D. Method for the solid-phase parallel synthesis of a 6-alkylamino-2-(functionalized-aminomethyl)-2H-1-benzopyran library. J. Comb. Chem. 2006, 8, 897–906. [Google Scholar] [CrossRef]
- Fernandez-Forner, D.; Huerta, J.M.; Ferrer, M.; Casals, G.; Ryder, H.; Giralt, E.; Albericio, F. Solid-phase syntheses of N-substituted carbamates. Reaction monitoring by gel-phase 13C-NMR using a 13C enriched BAL-linker. Tetrahedron Lett. 2002, 43, 3543–3546. [Google Scholar]
- Alsina, J.; Yokum, T.S.; Albericio, F.; Barany, G. A modified Backbone Amide Linker (BAL) solid-phase peptide synthesis strategy accommodating prolyl, N-alkylamino acyl, or histidyl derivatives at C-terminus. Tetrahedron Lett. 2000, 41, 7277–7280. [Google Scholar] [CrossRef]
- Jensen, K.J.; Alsina, J.; Songster, M.F.; Vagner, J.; Albericio, F.; Barany, G. Backbone amide linker (BAL) strategy for solid-phase synthesis of C-terminal-modified and cyclic peptides. J. Am. Chem. Soc. 1998, 120, 5441–5452. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Doming, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Oprea, T.I. Property distribution of drug-related chemical databases. J. Comput. Aided Mol. Des. 2000, 14, 251–264. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-J.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Gong, Y.-D.; Cheon, H.-G.; Lee, T.; Bae, M.-S.; Kang, N.S. A novel 6-(2-methyl-2-alkylsubstituted-2H-chromen-6-yl)-amine derivatives and pharmacophore model as 5-lipoxygenase inhibitors. Bull. Korean Chem. Soc. 2011, 32, 3752–3755. [Google Scholar] [CrossRef]
- Kim, J.-H.; Gong, Y.-D.; Lee, G.-H.; Seo, J.-S. Solid-phase parallel synthesis of a novel N-[Alkylsulfonamido-spiro(2H-1-benzopyran-2,4-piperidine)-6-yl] substituted amide and amine drug-like libraries. Bull. Korean Chem. Soc. 2012, 33, 128–136. [Google Scholar]
- Radmark, O. 5-Lipoxygenase-derived leukotrienes: Mediators also of atherosclerotic inflammation. Arterioscler Thromb. Vasc. Biol. 2003, 23, 1140–1142. [Google Scholar] [CrossRef]
- van Deventer, S.J.H. Small therapeutic molecules for the treatment of inflammatory bowel disease. Gut 2002, 50, ii47–ii53. [Google Scholar] [CrossRef]
- Sampson, A.P. The leukotrienes: Mediators of chronic inflammation in asthma. Clin. Exp. Allergy 1996, 26, 995–1004. [Google Scholar] [CrossRef]
- Brooks, C.D.W.; Summers, J.B. Modulators of leukotriene biosynthesis and receptor activation. J. Med. Chem. 1996, 39, 2629–2654. [Google Scholar] [CrossRef]
- Israel, E.; Rubin, P.; Kemp, J.P.; Grossman, J.; Pierson, W.; Siegel, S.C.; Tinkelman, D.; Murray, J.J.; Busse, W.; Segal, A.T.; et al. The effect of inhibition of 5-lipoxygenase by zileuton in mild-to-moderate asthma. Ann. Intern. Med. 1993, 119, 1059–1066. [Google Scholar]
- Falgueyret, J.P.; Hutchinson, J.H.; Riendeau, D. Criteria for the identification of non-redox inhibitors of 5-lipoxgenase. Biochem. Pharmacol. 1993, 45, 978–981. [Google Scholar] [CrossRef]
- Lau, C.K.; Bèlanger, P.C.; Dufresne, C.; Scheigetz, J.; Therieu, M.; Fitzsimmons, B.; Young, R.N.; Ford-Hutchinson, A.W.; Riendeau, D.; Denis, D.; et al. Development of 2,3-dihydro-6-(3-phenoxypropyl)-2-(2-phenylethyl)-5-benzofuranol (L-670,630) as a potent and orally active inhibitor of 5-lipoxygenase. J. Med. Chem. 1992, 35, 1299–1318. [Google Scholar] [CrossRef]
- Cho, Y.S.; Song, J.S.; Huh, J.Y.; Kim, C.H.; Gong, Y.-D.; Cheon, H.G. Discovery of (2-fluoro-benzyl)-(2-methyl-2-phenethyl-2H-chromen-6-yl)-amine (KRH-102140) as an orally active 5-lipoxygenase inhibitor with activity in murine inflammation models. Pharmacology 2011, 87, 49–55. [Google Scholar] [CrossRef]
- Semenza, G.L. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov. Today 2007, 20, 853–859. [Google Scholar] [CrossRef]
- Berra, E.; Ginouvés, A.; Pouysségur, J. The hypoxia-inducible-factor hydroxylases bring fresh air into hypoxia signaling. EMBO Rep. 2006, 7, 41–45. [Google Scholar] [CrossRef]
- Kaelin, W.G. Proline hydroxylation and gene expression. Ann. Rev. Biochem. 2005, 74, 115–128. [Google Scholar] [CrossRef]
- Hirsila, M.; Koivunen, P.; Gunzler, V.; Kivirikko, K.I.; Myllyharju, J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J. Biol. Chem. 2003, 278, 30772–30780. [Google Scholar]
- Wang, G.L.; Jiang, B.H.; Semenza, G.L. Effect of protein kinase and phosphatase inhibitors on expression of hypoxia-inducible factor 1. Biochem. Biophys. Res. Commun. 1995, 216, 669–675. [Google Scholar] [CrossRef]
- Nepal, M.; Gong, Y.-D.; Park, Y.R.; Soh, Y. An activator of PHD2, KRH102140, decreases angiogenesis via inhibition of HIF-1α. Cell Biochem. Funct. 2011, 28, 1–9. [Google Scholar]
- Breitenbucher, J.G.; Hui, H.C. Titanium mediated reductive amination on solid support: Extending the utility of the 4-hydroxy-thiophenol linker. Tetrahedron Lett. 1998, 39, 8207–8210. [Google Scholar]
- Cho, H.; Katoh, S.; Sayama, S.; Murakami, K.; Nakanishi, H.; Kajimoto, Y.; Ueno, H.; Kawasaki, H.; Aisaka, K.; Uchida, I. Synthesis and selective coronary vasodilatory activity of 3,4-dihydro-2,2-bis(methoxymethyl)-2h-1-benzopyran-3-ol derivatives: Novel potassium channel openers. J. Med. Chem. 1996, 39, 3797–3805. [Google Scholar] [CrossRef]
- Johnson, C.R.; Zhang, B.; Fantauzzi, P.; Hocker, M.; Yager, K.M. Libraries of N-alkylaminoheterocycles from nucleophilic aromatic substitution with purification by solid supported liquid extraction. Tetrahedron 1998, 54, 4097–4106. [Google Scholar] [CrossRef]
- Braitenbucher, J.G.; Johnson, C.R.; Haight, M.; Phelan, J.C. Generation of a piperzine-2-carboxamide library: A practical application of the phenol-sulfide react and release linker. Tetrahedron Lett. 1998, 39, 1295–1298. [Google Scholar] [CrossRef]
- Gong, Y.-D.; Hwang, J.Y.; Chon, Y.S.; Seo, J.-S.; Yoo, S.-E. Solid-phase synthesis of benzopyran building blocks via highly efficient hydroxy-alkoxylation. Bull. Korean Chem. Soc. 2002, 23, 1658–1660. [Google Scholar] [CrossRef]
- Seo, J.-S.; Joo, Y.-H.; Yi, J.B.; Lee, E.J.; Lee, N.; Cho, Y.-B.; Kwak, W.J.; Hwang, J.Y.; Jeon, Y.S.; Jeon, H.S.; et al. Novel inhibitors of prolyl 4-hydroxylase; Solid-phase synthesis of 2,2-dimethyl-3,4-dialkoxy-substituted 6-aminobenzopyran derivatives. Bull. Korean Chem. Soc. 2006, 27, 909–917. [Google Scholar] [CrossRef]
- Chao, E.Y.; Minick, D.J.; Sternbach, D.D.; Shearer, B.G.; Collins, J.L. A novel method for the generation of nitrile oxides on solid phase: Application to the synthesis of substituted benzopyranoisoxazoles. Org. Lett. 2002, 4, 323–326. [Google Scholar] [CrossRef]
- Brummond, K.M.; Lu, J. Solid-phase synthesis of BRL 49653. J. Org. Chem. 1999, 65, 1723–1726. [Google Scholar] [CrossRef]
- Swayze, E.E. Secondary amide-based linkers for solid phase organic synthesis. Tetrahedron Lett. 1997, 38, 8465–8468. [Google Scholar] [CrossRef]
- Devraj, R.; Cushman, M. A versatile solid phase synthesis of lavendustin a and certain biologically active analogs. J. Org. Chem. 1996, 61, 9368–9373. [Google Scholar] [CrossRef]
- Hamper, B.C.; Dukesherer, D.R.; South, M.S. Solid-phase synthesis of proline analogs via a three component 1,3-dipolar cycloaddition. Tetrahedron Lett. 1996, 37, 3671–3674. [Google Scholar] [CrossRef]
- Castro, J.L.; Matassa, V.G.; Ball, R.G. Mitsunobu-like processes with a novel triphenylphosphine-cyclic sulfamide betaine. J. Org. Chem. 1994, 59, 2289–2291. [Google Scholar] [CrossRef]
- Park, S.O.; Kim, J.; Koh, M.; Park, S.B. Efficient parallel synthesis of privileged benzopyranylpyrazoles via regioselective condensation of b-keto aldehydes with hydrazines. J. Comb. Chem. 2009, 11, 315–326. [Google Scholar] [CrossRef]
- Mock, W.L.; Tsou, H.R. A procedure for diethoxymethylation of ketones. J. Org. Chem. 1981, 46, 2557–2561. [Google Scholar] [CrossRef]
- Dasgupta, R.; Ghatak, U.R. A simple synthesis of a,b-unsaturated aldehydes by 1,3-carbonyl transposition through one carbon homologation. Tetrahedron Lett. 1985, 26, 1581–1584. [Google Scholar] [CrossRef]
- Sun, J.; Dong, Y.; Cao, L.; Wang, X.; Wang, S.; Hu, Y. Highly efficient chemoselective deprotection of o,o-acetals and o,o-ketals catalyzed by molecular iodine in acetone. J. Org. Chem. 2004, 69, 8932–8934. [Google Scholar] [CrossRef]
- Sagar, R.; Kim, M.-J.; Park, S.B. An improved synthesis of pyrimidine- and pyrazole-based acyclo-C-nucleosides as carbohybrids. Tetrahedron Lett. 2008, 49, 5080–5083. [Google Scholar] [CrossRef]
- Sagar, R.; Park, S.B. Facile and efficient synthesis of carbohybrids as stereodivergent druglike small molecules. J. Org. Chem. 2008, 73, 3270–3273. [Google Scholar] [CrossRef]
- Lee, S.-C.; Park, S.B. Novel application of Leuckart-Wallach reaction for synthesis of tetrahydro-1,4-benzodiazepin-5-ones library. Chem. Commun. 2007, 3714–3716. [Google Scholar]
- Lee, S.-C.; Park, S.B. Practical solid-phase parallel synthesis of ∆5–2-oxopiperazines via N-acyliminium ion cyclization. J. Comb. Chem. 2007, 9, 828–835. [Google Scholar] [CrossRef]
- Lee, S.-C.; Choi, S.Y.; Chung, Y.K.; Park, S.B. Preparation of pilot library with tetrahydro-b-carboline alkaloid core skeleton using tandem intramolecular Pictet-Spengler cyclization. Tetrahedron Lett. 2006, 47, 6843–6847. [Google Scholar]
- Ko, S.K.; Jang, H.J.; Kim, E.; Park, S.B. Concise and diversity-oriented synthesis of novel scaffolds embedded with privileged benzopyran motif. Chem. Commun. 2006, 2962–2964. [Google Scholar]
- Lee, S.-C.; Park, S.B. Solid-phase parallel synthesis of natural product-like diaza-bridged heterocycles through pitet-spengler intramolecular cyclization. J. Comb. Chem. 2006, 8, 50–57. [Google Scholar] [CrossRef]
- Sanchez, I.H.; Lopez, F.J.; Soria, J.J.; Larraza, M.I.; Flores, H.J. Total synthesis of (±)-elwesine, (±)-epielwesine, and (±)-oxocrinine. J. Am. Chem. Soc. 1983, 105, 7640–7643. [Google Scholar] [CrossRef]
- Kapeller, D.C.; Bräse, S. Versatile solid-phase synthesis of chromenes resembling classical cannabinoids. ACS Comb. Sci. 2011, 13, 554–561. [Google Scholar] [CrossRef]
- Thompson, L.A.; Ellman, J.A. Straightforward and general method for coupling alcohols to solid supports. Tetrahedron Lett. 1994, 35, 9333–9336. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Chen, F. Solid-phase synthesis of solanesol. J. Comb. Chem. 2008, 10, 605–610. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Kapeller, D.C.; Bräse, S. Towards a library of chromene cannabinoids: A combinatorial approach on solid supports. Synlett 2011, 161–164. [Google Scholar]
- Lesch, B.; Toräng, J.; Nieger, M.; Bräse, S. The Diels-Alder approach towards cannabinoids. Synthesis 2005, 1888–1900. [Google Scholar]
- Lesch, B.; Toräng, J.; Vanderheiden, S.; Bräse, S. Base-catalyzed condensation of 2-hydroxybenzaldehyes with a,β-unsaturated aldehydes: Scope and limitations. Adv. Synth. Catal. 2005, 347, 555–562. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.-Y.; Brossi, A.; Lee, K.-H. Asymmetric solid-phase synthesis of (3'R,4'R)-di-O-cis-acyl 3-carboxyl khellactones. Org. Lett. 1999, 1, 2113–2115. [Google Scholar] [CrossRef]
- Xie, L.; Takeuchi, Y.; Cosentino, L.M.; Lee, K.-H. Anti-AIDS Agents. 37. Synthesis and structure-activity relationships of (3'R,4'R)-(+)-cis-khellactone derivatives as novel potent anti-HIV agents. J. Med. Chem. 1999, 42, 2662–2672. [Google Scholar]
- Watson, B.T.; Christiansen, G.E. Solid phase synthesis of substituted coumarin-3-carboxylic acids via the knoevenagel condensation. Tetrahedron Lett. 1998, 39, 6087–6090. [Google Scholar] [CrossRef]
- Hamper, B.C.; Kolodziej, S.A.; Scates, A.M. Knoevenagel condensation of unsymmetrical malonamic esters and malonates on a solid support. Tetrahedron Lett. 1998, 39, 2047–2050. [Google Scholar]
- Gordeev, M.F.; Patel, D.V.; Wu, J.; Gordon, E.M. Approaches to combinatorial synthesis of heterocycles: Solid phase synthesis of pyridines and pyrido[2,3-d]pyrimidines. Tetrahedron Lett. 1996, 37, 4643–4646. [Google Scholar] [CrossRef]
- Zaragoza, F. Carbon-carbon bond formation on solid support: Synthesis of monoacyl piperazines by knoevenagel-type condensation reactions. Tetrahedron Lett. 1995, 36, 8677–8678. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pfefferkorn, J.A.; Cao, G.-Q. Selenium-based solid-phase syntesis of benzopyrans I: Applications to combinatorial synthesis of natural products. Angew. Chem. Int. Ed. Engl. 2000, 39, 734–739. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Cao, G.-Q.; Pfefferkorn, J.A. Selenium-based solid-phase synthesis of benzopyrans ii: Applications to combinatorial synthesis of medicinally relevant small organic molecules. Angew. Chem. Int. Ed. Engl. 2000, 39, 739–743. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pfefferkorn, J.A.; Roecker, A.J.; Cao, G.-Q.; Barluenga, S.; Mitchell, H.J. Natural product-like combinatorial libraries based on privileged structures. 1. General principles and solid-phase synthesis of benzopyrans. J. Am. Chem. Soc. 2000, 122, 9939–9953. [Google Scholar]
- Nicolaou, K.C.; Pfefferkorn, J.A.; Mitchell, H.J.; Roecker, A.J.; Barluenga, S.; Cao, G.-Q.; Affleck, R.L.; Lillig, J.E. Natural product-like combinatorial libraries based on privileged structures. 2. construction of a 10 000-membered benzopyran library by directed split-and-pool chemistry using NanoKans and optical encoding. J. Am. Chem. Soc. 2000, 122, 9954–9967. [Google Scholar]
- Nicolaou, K.C.; Pfefferkorn, J.A.; Barluenga, S.; Mitchell, H.J.; Roecker, A.J.; Cao, G.-Q. Natural product-like combinatorial libraries based on privileged structures. 3. The “Libraries from Libraries” principle for diversity enhancement of benzopyran libraries. J. Am. Chem. Soc. 2000, 122, 9968–9976. [Google Scholar]
- Nicolaou, K.C.; Pfefferkorn, J.A.; Schuler, F.; Roecker, A.J.; Cao, G.Q.; Casida, J.E. Combinatorial synthesis of novel and potent inhibitors of NADH:ubiquinone oxidoreductase. Chem. Biol. 2000, 7, 979–992. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Roecker, A.J.; Barluenga, S.; Pfefferkorn, J.A.; Cao, G.Q. Discovery of novel antibacterial agents active against methicillin-resistant staphylococcus aureus from combinatorial benzopyran libraries. ChemBioChem 2001, 460–465. [Google Scholar]
- Ruhland, T.; Anderson, K.; Pedersen, H. Selenium-linking strategy for traceless solid-phase synthesis: Direct loading, aliphatic C–H bond formation upon cleavage and reaction monitoring by gradient MAS NMR spectroscopy. J. Org. Chem. 1998, 63, 9204–9211. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pastor, J.; Barluenga, S.; Winssinger, N. Polymer-supported selenium reagents for organic synthesis. Chem. Commun. 1998, 1947–1948. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, T.; Gong, Y.-D. Solid-Phase Parallel Synthesis of Drug-Like Artificial 2H-Benzopyran Libraries. Molecules 2012, 17, 5467-5496. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules17055467
Lee T, Gong Y-D. Solid-Phase Parallel Synthesis of Drug-Like Artificial 2H-Benzopyran Libraries. Molecules. 2012; 17(5):5467-5496. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules17055467
Chicago/Turabian StyleLee, Taeho, and Young-Dae Gong. 2012. "Solid-Phase Parallel Synthesis of Drug-Like Artificial 2H-Benzopyran Libraries" Molecules 17, no. 5: 5467-5496. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules17055467