Antimicrobial Activity of UV-Induced Phenylamides from Rice Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenylamides Accumulate in UV-Treated Rice Leaves


2.2. UV Irradiation Stimulates Phenylamide Biosynthetic Pathways in Rice Leaves

2.3. UV-Induced Phenylamides in Rice Leaves are Potential Phytoalexins against Rice Pathogens
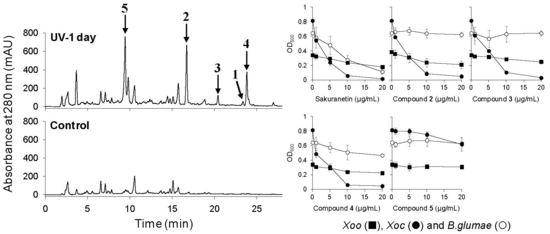
2.3.1. Antimicrobial Activity of UV-Induced Phenolic Compounds
| Compound | Fungal Pathogen | Bacterial Pathogen | ||||
|---|---|---|---|---|---|---|
| M. grisea | R. solani | B. oryzae | Xoo | Xoc | B. glumae | |
| 1 | 6.44 ± 1.35 | 54.04 ± 22.61 | 19.05 ± 9.83 | 19.95 ± 6.20 | 2.36 ± 0.35 | 8.22 ± 1.49 |
| 2 | - a | - | - | 21.96 ± 7.00 | 3.18 ± 0.44 | - |
| 3 | - | - | - | 34.76 ± 10.98 | 3.72 ± 0.20 | - |
| 4 | - | - | 26.92 ± 4.74 | 24.34 ± 5.60 | 2.45 ± 0.48 | 41.09 ± 5.96 |
| 5 | - | - | - | - | 54.54 ± 20.84 | - |

2.3.2. UV-Induced Phenylamides Are Potential Phytoalexins in Rice
3. Experimental Section
3.1. Plant Growth Conditions and UV Treatment
3.2. Analysis, Isolation and Identification of Phenylamides from UV-Treated Rice Leaves
3.3. RNA Isolation and Semi-Quantitative RT-PCR Analysis
3.4. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cartwright, D.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Chemical activation of host defense mechanisms as a basis for crop protection. Nature 1997, 267, 511–513. [Google Scholar] [CrossRef]
- Cartwright, D.W.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 1981, 20, 535–537. [Google Scholar] [CrossRef]
- Akatsuka, T.; Kodama, O.; Sekido, H.; Kono, Y.; Takeuchi, S. Novel phytoalexins (Oryzalexins A, B, and C) isolated from rice blast leaves infected with Pyricularia oryzae. Part I: Isolation, characterization and biological activities of oryzalexins. Agric. Biol. Chem. 1985, 49, 1689–1694. [Google Scholar]
- Sekido, H.; Endo, T.; Suga, R.; Kodama, O.; Akatsuka, T.; Kono, Y.; Takeuchi, S. Oryzalexin D (3,7-(+)-sandaracopimaradiene), a new phytoalexin isolated from blast-infected rice leaves. J. Pesticide Sci. 1986, 11, 369–372. [Google Scholar] [CrossRef]
- Kodama, O.; Suzuki, T.; Miyakawa, J.; Akatsuka, T. Ultraviolet-induced accumulation of phytoalexins in rice leaves. Agric. Biol. Chem. 1988, 52, 2469–2473. [Google Scholar] [CrossRef]
- Kodama, O.; Miyakawa, J.; Akatsuka, T.; Kiyosawa, S. Sakuranetin, a flavanone phytoalexin from ultraviolet-irradiated rice leaves. Phytochemistry 1992, 31, 3807–3809. [Google Scholar] [CrossRef]
- Kato, H.; Kodama, O.; Akatsuka, T.; Akatsuka, T. Oryzalexin E, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry 1993, 33, 79–81. [Google Scholar] [CrossRef]
- Kato, H.; Kodama, O.; Akatsuka, T. Oryzalexin F, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry 1994, 36, 299–301. [Google Scholar] [CrossRef]
- Koga, J.; Shimura, M.; Oshima, K.; Ogawa, N.; Yamauchi, T.; Ogasawara, N. Phytocassanes A, B, C and D, novel diterpene phytoalexins from rice, Oryza sativa L. Tetrahedron 1995, 51, 7907–7918. [Google Scholar] [CrossRef]
- Koga, J.; Ogawa, N.; Yamauchi, T.; Kikuchi, M.; Ogasawara, N.; Shimura, M. Functional moiety for the antifungal activity of phytocassane E, a diterpene phytoalexin from rice. Phytochemistry 1997, 44, 249–253. [Google Scholar] [CrossRef]
- Okada, A.; Shimizu, T.; Okada, K.; Kuzuyama, T.; Koga, J.; Shibuya, N.; Nojiri, H.; Yamane, H. Elicitor induced activation of the methylerythritol phosphate pathway toward phytoalexins biosynthesis in rice. Plant Mol. Biol. 2007, 65, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Sakai, M.; Yao, Q.; Tanimoto, Y.; Toshima, H.; Hasegawa, M. Identification of a novel casbane-type diterpene phytoalexin, ent-10-oxodepressin, from rice leaves. Biosci. Biotechnol. Biochem. 2013, 77, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Kato, Y.; Kitahara, Y. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 39, 3861–3864. [Google Scholar] [CrossRef]
- Cho, E.M.; Okada, A.; Kenmoku, H.; Otomo, K.; Toyomasu, T.; Mitsuhashi, W.; Sassa, T.; Yajima, A.; Yabuta, G.; Mori, K.; et al. Molecular cloning and characterization of cDNA encoding ent-cassa-12,15-diene synthase, a putative diterpenoid phytoalexin biosynthetic enzyme, from suspension-cultured rice cells treated with a chitin elicitor. Plant J. 2004, 37, 1–8. [Google Scholar]
- Nemoto, T.; Cho, E.M.; Okada, A.; Okada, K.; Omoto, K.; Kanno, Y.; Toyomasu, T.; Mitsuhashi, W.; Sassa, T.; Minami, E.; et al. Stemar-13-ene synthase, a diterpene cyclase involved in the biosynthesis of the phytoalexin oryzalexin S in rice. FEBS Lett. 2004, 571, 182–186. [Google Scholar]
- Okada, K. The biosynthesis of isoprenoids and the mechanisms regulating it in plants. Biosci. Biotechnol. Biochem. 2011, 75, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J. Uncovering the complex metabolic network underlying diterpenoid phytoalexin biosynthesis in rice and other cereal crop plants. Phytochemistry 2006, 67, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, T. Recent advances regarding diterpene cyclase genes in higher plants and fungi. Biosci. Biotechnol. Biochem. 2008, 72, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014, 79, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Edreva, A.M.; Velikova, V.B.; Tsonev, T.D. Phenylamides in plants. Russ. J. Plant Physiol. 2007, 54, 287–301. [Google Scholar] [CrossRef]
- Bassard, J.E.; Ullmann, P.; Bernier, F.; Werck-Reichhart, D. Phenolamides: Bridging polyamines to the phenolic metabolism. Phytochemistry 2010, 71, 1808–1824. [Google Scholar] [CrossRef] [PubMed]
- Klapheck, S. Polyamines and cinnamoyl-putrescines in normal and sulfur-starved suspension cultures of Nicotiana tabacum. Z. Pflanzenphysiol. 1983, 112, 275–279. [Google Scholar] [CrossRef]
- Edreva, A.; Yordanov, I.; Kardjieva, R.; Hadjiiska, E.; Gesheva, E. Expression of phenylamides in abiotic stress conditions. Bulg. J. Plant Physiol. 1995, 21, 15–23. [Google Scholar]
- Pearce, G.; Marchand, P.A.; Griswold, J.; Lewis, N.G.; Ryan, C.A. Accumulation of feruloyltyramine and p-coumaroyltyramine in tomato leaves in response wounding. Phytochemistry 1998, 47, 659–664. [Google Scholar] [CrossRef]
- Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O. Antifungal N-feruloyl amides from roots of two Allium species. Plant Biosyst. 1999, 133, 199–203. [Google Scholar] [CrossRef]
- Newman, M.A.; von Roepenack-Lahaye, E.; Parr, A.; Daniels, M.J.; Dow, J.M. Induction of hydroxycinnamoyl-tyramine conjugates in pepper by Xanthomonas campestris, a plant defense response activated by hrp gene-dependent and hrp gene-independent mechanisms. MPMI 2001, 14, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Tanaka, C.; Mori, N.; Kuwahara, Y.; Tsuda, M. Phenylpropanoid amides of serotonin accumulate in witches’ broom diseased bamboo. Phytochemistry 2003, 64, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Von Roepenack-Lahaye, E.; Newman, M.A.; Schornack, S.; Hammond-Kosack, K.E.; Lahaye, T.; Jones, J.D.G.; Daniels, M.J.; Dow, J.M. p-Coumaroylnoradrenaline, a novel plant metabolite implicated in tomato defense against pathogens. J. Biol. Chem. 2003, 278, 43373–73383. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Nakao, T.; Mashimo, Y.; Murai, M.; Ichimaru, N.; Tanaka, C.; Nakajima, H.; Wakasa, K.; Miyagawa, H. Probing the role of tryptophan-derived secondary metabolism in defense responses against Bipolaris oryzae infection in rice leaves by a suicide substrate of tryptophan decarboxylase. Phytochemistry 2011, 72, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Hashmoto, Y.; Tanaka, C.; Dubouzet, J.G.; Nakao, T.; Matsuda, F.; Nishioka, T.; Miyagawa, H.; Wakasa, K. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Lee, S.W.; Jung, K.H.; Hahn, T.R.; Cho, M.H. Transcriptomic analysis of UV-treated rice leaves reveals UV-induced phytoalexin biosynthetic pathways and their regulatory networks in rice. Phytochemistry 2013, 96, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Fu, Y.Y.; Chung, I.S.; Hahn, T.R.; Cho, M.H. Cytotoxic property of ultraviolet-induced rice phytoalexins to human colon carcinoma HCT-116 cells. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 237–241. [Google Scholar] [CrossRef]
- Sakamura, S.; Terayama, Y.; Kawakatsu, S.; Ichihara, A.; Saito, H. Conjugated serotonins related to cathartic activity in safflower seeds (Carthamus tinctorius L.). Agric. Biol. Chem. 1978, 42, 1805–1806. [Google Scholar] [CrossRef]
- Quinet, M.; Ndayiragije, A.; Lefèvre, I.; Lambillotte, B.; Dupont-Gillain, C.C.; Lutts, S. Putrescine differently influences the effect of salt stress on polyamine metabolism and ethylene synthesis in rice cultivars differing in salt resistance. J. Exp. Bot. 2010, 61, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Kasai, K.; Ikejiri-Kanno, Y.; Wakasa, K.; Tozawa, Y. In vitro reconstitution of rice anthranilate synthase: Distinct functional properties of the α subunits OASA1 and OASA2. Plant Mol. Biol. 2004, 54, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kang, K.; Lee, K.; Back, K. Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 2007, 227, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sekido, H.; Akatsuka, T. Mode of action of oryzalexin D against Pyricularia oryzae. Agric. Biol. Chem. 1987, 51, 1967–1971. [Google Scholar] [CrossRef]
- Sample Availability: Samples of sakuranetin are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.L.; Yoo, Y.; Hahn, T.-R.; Bhoo, S.H.; Lee, S.-W.; Cho, M.-H. Antimicrobial Activity of UV-Induced Phenylamides from Rice Leaves. Molecules 2014, 19, 18139-18151. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules191118139
Park HL, Yoo Y, Hahn T-R, Bhoo SH, Lee S-W, Cho M-H. Antimicrobial Activity of UV-Induced Phenylamides from Rice Leaves. Molecules. 2014; 19(11):18139-18151. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules191118139
Chicago/Turabian StylePark, Hye Lin, Youngchul Yoo, Tae-Ryong Hahn, Seong Hee Bhoo, Sang-Won Lee, and Man-Ho Cho. 2014. "Antimicrobial Activity of UV-Induced Phenylamides from Rice Leaves" Molecules 19, no. 11: 18139-18151. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules191118139