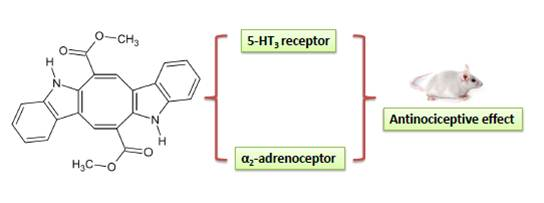
Assessment of Mechanisms Involved in Antinociception Produced by the Alkaloid Caulerpine
Abstract
:1. Introduction
2. Results and Discussion






3. Experimental Section
3.1. Animals
3.2. Drugs
3.3. Isolation of Caulerpine
3.4. HPLC Analysis
3.5. Mechanism of the Antinociceptive Action of Caulerpine
3.5.1. Involvement of the Opioid, l-Arginine-Nitric Oxide, Adrenergic, Cholinergic, GABAergic and Serotonergic Pathways
| Pathway | Pretreatment | Positive Control | Reference |
|---|---|---|---|
| Opiod | Naloxone (1 mg/kg, i.p.) | Morphine (5 mg/kg, i.p.) | [31] |
| Adrenergic (α2) | Yohimbine (0.15 mg/kg, i.p.) | Clonidine (0.1 mg/kg, i.p.) | [25] |
| Cholinergic (muscarinic) | Atropine (1 mg/kg, i.p.) | Pilocarpine (3 mg/kg, i.p.) | [32] |
| GABAergic (GABAA) | Flumazenil (2 mg/kg, i.p | Diazepam (1.5 mg/kg, i.p.) | [33] |
| Serotonergic (5-HT3) | Tropisetron (1 mg/kg, i.p.) | 1-(3-chlorophenyl)biguanide (1 mg/kg, i.p.) | [25] |
| l-arginine-NO | l-arginine (40 mg/kg, i.p.) | l-NOARG (75 mg/kg, i.p.) | [25] |
3.5.2. α2-Adrenoceptor Binding Assay
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef]
- Souza, E.T.; Lira, D.P.; Queiroz, A.C.; Silva, D.J.C.; Aquino, A.B.; Mella, E.A.C.; Lorenzo, V.L.; Miranda, G.E.C.; Araújo-Júnior, J.X.; Chaves, M.C.O.; et al. The antinociceptive and anti-inflammatory activities of caulerpine, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef]
- Souza, E.T.; Queiroz, A.C.; Miranda, G.E.C.; Lorenzo, V.P.; Silva, E.F.; Freire-Dias, T.L.M.; Cupertino-Silva, Y.K.; Melo, G.M.A.; Chaves, M.C.O.; Barbosa-Filho, J.M.; et al. Antinociceptive activities of crude methanolic extract and phases, n-butanolic, chloroformic and ethyl acetate from Caulerpa racemosa (Caulerpaceae). Braz. J. Pharmacogn. 2009, 19, 115–120. [Google Scholar] [CrossRef]
- Matta, C.B.B.; Souza, E.T.; Queiroz, A.C.; Lira, D.P.; Araújo, M.V.; Cavalcante-Silva, L.H.A.; Miranda, G.E.C.; Araújo-Júnior, J.X.; Barbosa-Filho, J.M.; Santos, B.V.O.; et al. Antinociceptive and anti-inflammatory activity from algae of the genus Caulerpa. Mar. Drugs 2011, 9, 307–318. [Google Scholar] [CrossRef]
- Cavalcante-Silva, L.H.A.; de Carvalho Correia, A.C.; Barbosa-Filho, J.M.; da Silva, B.A.; de Oliveira Santos, B.V.; de Lira, D.P.; Sousa, J.C.F.; de Miranda, G.E.C.; de Andrade Cavalcante, F.; Alexandre-Moreira, M.S. Spasmolytic effect of caulerpine involves blockade of Ca2+ influx on guinea pig ileum. Mar. Drugs 2013, 11, 1553–1564. [Google Scholar] [CrossRef]
- Aguilar-Santos, G. Caulerpine, a new red pigment from green algae of the genus Caulerpa. J. Chem. Soc. C 1970, 6, 1842–1843. [Google Scholar]
- Anjaneyulu, A.S.R.; Prakash, C.V.S.; Mallavadhani, U.V. Sterols and terpenes of the marine green algal species Caulerpa racemosa and Codium decorticatum. J. Indian Chem. Soc. 1991, 68, 480. [Google Scholar]
- Yan, S.; Su, J.; Wang, Y.; Zeng, L. Studies on chemical constituents of Halimeda incrassata. Trop. Ocean 1999, 18, 91–94. [Google Scholar]
- Govenkar, M.B.; Wahidulla, S. Constituents of Chondria armata. Phytochemistry 2002, 54, 979–981. [Google Scholar] [CrossRef]
- Cunha, T.M.; Roman-Campos, D.; Lotufo, C.M.; Duarte, H.L.; Souza, G.R.; Verri, W.A., Jr.; Funez, M.I.; Dias, Q.M.; Schivo, I.R.; Domingues, A.C.; et al. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4442–4447. [Google Scholar] [CrossRef]
- Bodnar, R.J. Endogenous opiates and behavior: 2011. Peptides 2012, 38, 463–522. [Google Scholar] [CrossRef]
- Jones, P.G.; Dunlop, J. Targeting the cholinergic system as a therapeutic strategy for the treatment of pain. Neuropharmacology 2007, 53, 197–206. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef]
- Sommer, C. Serotonin in pain and analgesia. Mol. Neurobiol. 2004, 30, 117–125. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Yamazaki, H.; Hori, Y. Enkephalinergic neurons express 5-HT3 receptors in the spinal cord dorsal horn: Single cell RT-PCR analysis. Neuroreport 1999, 10, 2749–2753. [Google Scholar] [CrossRef]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Duarte, I.D.G.; Nakamura, M.; Ferreira, S.H. Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz. J. Med. Biol. Res. 1988, 21, 341–343. [Google Scholar]
- Ikeda, Y.; Ueno, A.; Naraba, H.; Oh-Ishi, S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001, 69, 2911–2919. [Google Scholar] [CrossRef]
- Souza, G.F.P.; Zarpelon, A.C.; Tedeschi, G.C.; Mizokami, S.S.; Sanson, J.S.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Casagrande, R.; Verri, W.A., Jr. Acetic acid- and phenyl-p-benzoquinone-induced overt pain-like behavior depends on spinal activation of MAP kinases, PI(3)K and microglia in mice. Pharmacol. Biochem. Behav. 2012, 101, 320–328. [Google Scholar] [CrossRef]
- Shamima, A.R.; Fakurazi, S.; Hidayat, M.T.; Hairuszah, I.; Moklas, M.A.M.; Arulselvan, P. Antinociceptive action of isolated mitragynine from Mitragyna speciosa through activation of opioid receptor system. Int. J. Mol. Sci. 2012, 13, 11427–11442. [Google Scholar] [CrossRef]
- Matsumoto, K.; Horie, S.; Ishikawa, H.; Takayama, H.; Aimi, N.; Ponglux, D.; Watanabe, K. Antinociceptive effect of 7-hydroxymitragynine in mice: Discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2004, 74, 2143–2155. [Google Scholar] [CrossRef]
- Santos, A.R.S.; Gadotti, V.M.; Oliveira, G.L.; Tibola, D.; Paszcuk, A.F.; Neto, A.; Spindola, H.M.; Souza, M.M.; Rodrigues, A.L.; Calixto, J.B. Mechanism involved in the antinociception caused by agmatine in mice. Neuropharmacology 2005, 48, 1021–1034. [Google Scholar] [CrossRef]
- Lin, M.T.; Su, C.F. Spinal 5-HT pathways and the antinociception induced by intramedullary clonidine in rats. Naunyn Schmiedebergs Arch. Pharmacol. 1992, 346, 333–338. [Google Scholar]
- Fox, A.; Morton, I.K. An examination of the 5-HT3 receptor mediating contraction and evoked [3H]-acetylcholine release in the guinea-pig ileum. Br. J. Pharmacol. 1990, 101, 553–558. [Google Scholar] [CrossRef]
- Tuladhar, B.R.; Kaisar, M.; Naylor, R.J. Evidence for a 5-HT3 receptor involvement in the facilitation of peristalsis on mucosal application of 5-HT in the guinea pig isolated ileum. Br. J. Pharmacol. 1997, 122, 1174–1178. [Google Scholar] [CrossRef]
- Hu, J.F.; Schetz, J.A.; Kelly, M.; Peng, J.N.; Ang, K.K.; Flotow, H.; Leong, C.Y.; Ng, S.B.; Buss, A.D.; Wilkins, S.P.; et al. New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. J. Nat. Prod. 2002, 65, 476–480. [Google Scholar] [CrossRef]
- Collier, H.O.J.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The abdominal constriction response and its suppression by analgesic drugs in mice. Br. J. Pharmacol. 1968, 32, 285–310. [Google Scholar]
- Mantovani, M.; Kaster, M.P.; Pertile, R.; Calixto, J.B.; Rodrigues, A.L.; Santos, A.R. Mechanisms involved in the antinociception caused by melatonin in mice. J. Pineal Res. 2006, 41, 382–389. [Google Scholar] [CrossRef]
- Guginski, G.; Luiz, A.P.; Silva, M.D.; Massaro, M.; Martins, D.F.; Chaves, J.; Mattos, R.W.; Silveira, D.; Ferreira, V.M.; Calixto, J.B.; et al. Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol. Biochem. Behav. 2009, 93, 10–16. [Google Scholar]
- Talarek, S.; Fidecka, S. Role of nitric oxide in benzodiazepines-induced antinociception in mice. Pol. J. Pharmacol. 2002, 54, 27–34. [Google Scholar]
- Pompeu, T.E.T.; Alves, F.R.; Figueiredo, C.D.; Antonio, C.B.; Herzfeldt, V.; Moura, B.C.; Rates, S.M.; Barreiro, E.J.; Fraga, C.A.; Noël, F. Synthesis and pharmacological evaluation of new N-phenylpiperazine derivatives designed as homologues of the antipsychotic lead compound LASSBio-579. Eur. J. Med. Chem. 2013, 66, 122–134. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cavalcante-Silva, L.H.A.; Falcão, M.A.P.; Vieira, A.C.S.; Viana, M.D.M.; De Araújo-Júnior, J.X.; Sousa, J.C.F.; Silva, T.M.S.d.; Barbosa-Filho, J.M.; Noël, F.; De Miranda, G.E.C.; et al. Assessment of Mechanisms Involved in Antinociception Produced by the Alkaloid Caulerpine. Molecules 2014, 19, 14699-14709. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules190914699
Cavalcante-Silva LHA, Falcão MAP, Vieira ACS, Viana MDM, De Araújo-Júnior JX, Sousa JCF, Silva TMSd, Barbosa-Filho JM, Noël F, De Miranda GEC, et al. Assessment of Mechanisms Involved in Antinociception Produced by the Alkaloid Caulerpine. Molecules. 2014; 19(9):14699-14709. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules190914699
Chicago/Turabian StyleCavalcante-Silva, Luiz Henrique Agra, Maria Alice Pimentel Falcão, Ana Carolina Santana Vieira, Max Denisson Maurício Viana, João Xavier De Araújo-Júnior, Jéssica Celestino Ferreira Sousa, Tania Maria Sarmento da Silva, José Maria Barbosa-Filho, François Noël, George Emmanuel C. De Miranda, and et al. 2014. "Assessment of Mechanisms Involved in Antinociception Produced by the Alkaloid Caulerpine" Molecules 19, no. 9: 14699-14709. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules190914699