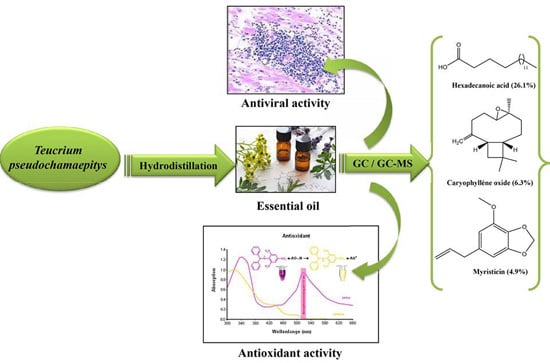
Essential Oil Composition, Antioxidant, Cytotoxic and Antiviral Activities of Teucrium pseudochamaepitys Growing Spontaneously in Tunisia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
| Compound | RI | % | Identification |
|---|---|---|---|
| Trans-sabinene hydrate | 1058 | 0.9 | RI,MS |
| Borneol | 1131 | 1.8 | RI,MS |
| Terpin-4-ol | 1148 | 0.3 | RI,MS |
| α-Terpineol | 1161 | 0.5 | RI,MS |
| Thymol | 1282 | 2.9 | RI,MS |
| Carvacrol | 1292 | 1.5 | RI,MS |
| Durenol | 1325 | 0.4 | RI,MS |
| α-Cubebene | 1373 | 3.9 | RI,MS |
| E-β-Damascenone | 1391 | 4.6 | RI,MS |
| α-Copaene | 1404 | 1.0 | RI,MS |
| β-Bourbonene | 1412 | 0.8 | RI,MS |
| β-Caryophyllene | 1450 | 3.5 | RI,MS |
| β-Humulene | 1473 | 0.5 | RI,MS |
| α-Humulene | 1489 | 1.7 | RI,MS |
| Dehydro-Aromadendrene | 1487 | 1.3 | RI,MS |
| E-β-Ionone | 1510 | 0.3 | RI,MS |
| γ-Muurolene | 1511 | 2.8 | RI,MS |
| Germacrene D | 1520 | 0.7 | RI,MS |
| α-Selinene | 1525 | 0.3 | RI,MS |
| Myristicin | 1539 | 4.9 | RI,MS |
| δ-Cadinene | 1556 | 2.1 | RI,MS |
| Cadine-1,4-diene | 1568 | 3.1 | RI,MS |
| α-Cadinene | 1580 | 1.2 | RI,MS |
| Elemicin | 1584 | 3.3 | RI,MS |
| 1-nor-Bourbonanone | 1589 | 0.2 | RI,MS |
| Pentyl salicylate | 1609 | 0.4 | RI,MS |
| Caryophyllene oxide | 1636 | 6.3 | RI,MS |
| Apiole | 1717 | 7.1 | RI,MS |
| Myristic acid | 1833 | 1.1 | RI,MS |
| Pentadecanol | 1873 | 3.1 | RI,MS |
| Hexadecanoic acid | 2030 | 26.1 | |
| Oxygenated monoterpenes | 3.5 | ||
| Sesquiterpene hydrocarbons | 27.8 | ||
| Oxygenated sesquiterpene | 6.3 | ||
| Other | 51 | ||
| Total identified | 88.6 | ||
2.2. DPPH Radical Scavenging Activity
| DPPH Inhibition | |||||
|---|---|---|---|---|---|
| Concentration | 1 mg·mL−1 | 0.5 mg·mL−1 | 0.25 mg·mL−1 | 0.125 mg·mL−1 | IC50 |
| Essential oil | 51.89 ± 0.00 | 44.93 ± 1.90 | 35.43 ± 1.24 | 19.64 ± 3.19 | 0.77 ± 0.05 |
| Quercetin | 96.42 ± 0.45 | 92.57 ± 0.17 | 90 ± 0.72 | 89.57 ± 0.16 | 0.069 ± 0.02 |
2.3. Cell Viability Test

2.4. Inhibition of CV-B Infectivity by Teucrium Essential Oil
| CC50 (µg·mL−1) | IC50 (µg·mL−1) | SI | CC80 (µg·mL−1) | IC80 (µg·mL−1) | |
|---|---|---|---|---|---|
| Teucrium essential oil | 653.6 ± 0.11 | 589.6 ± 0.46 | 1.11 | 2534.6 ± 0.08 | 852.3 ± 0.67 |

3. Experimental Section
3.1. Plant Material and Isolation of Essential Oil
3.2. Gas Chromatography
3.3. Gas Chromatography-Mass Spectrometry
3.4. Identification of the Components
3.5. Antioxidant Activity
3.6. Viruses and Cell Line
3.7. Cell Seeding and Infection of Cell Cultures
3.8. Cytotoxicity Assay
3.9. Cytopathic Effect Inhibition Assay
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bhuwan, K.C.; Nasser, A.; Awadh, A.; William, N.S. A survey of chemical compositions and biological activities of Yemeni Aromatic Medicinal Plants. Medicines 2015, 2, 67–92. [Google Scholar] [CrossRef]
- Hussein, A.H.; Said, A.A.; Sarhan, A.M.; Abou Dahab, A.D.M.; Abou-Zeid, E.S.N.; Ali, M.S.; Naguib, N.Y.; el Bendary, M.A. Essential oils of Anethum graveolens L.: Chemical composition and their antimicrobial activities at vegetative, flowering and fruiting stages of development. Int. J. Plant Sci. Ecol. 2015, 1, 98–102. [Google Scholar]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid. Based Complement. Altern. Med. 2015, 2015, 397821. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, E.; Boulos, L.; Véla, E. Catalogue Synonymique Commenté de la Flore de Tunisie, 2nd ed.; Banque Nationale de Gènes de la Tunisie: Tunis, Tunisie, 2010. [Google Scholar]
- Radulović, N.; Dekić, M.; Joksović, M.; Vukićević, R. Chemotaxonomy of Serbian Teucrium species inferred from essential oil chemical composition: The case of Teucrium scordium L. ssp. Scordioides. Chem. Biodivers. 2012, 2, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Roghayeh, M.; Sepehri, Z.; Jahantigh, M.; Javadian, F. Antimicrobial activities of Teucrium polium against Salmonella typhimurium. Int. J. Adv. Biol. Biomed. Res. 2015, 3, 149–152. [Google Scholar]
- Bezić, N.; Vuko, E.; Dunkić, V.; Ruščić, M.; Blažević, I.; Burčul, F. Antiphytoviral activity of sesquiterpene-rich essential oils from four Croatian Teucrium species. Molecules 2011, 16, 8119–8129. [Google Scholar] [CrossRef] [PubMed]
- Pottier-Alapétite, G. Flore de la Tunisie. Dicotylédones, Gamopétales, Tunis; Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et Ministère de l’Agriculture: Tunis, Tunisie, 1981. [Google Scholar]
- Klingel, K.; Hohenadl, C.; Canu, A.; Albrecht, M.; Seemann, M.; Mall, G. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: Quantitative analysis of virus replication, tissue damage and inflammation. Proc. Natl. Acad. Sci. USA 1992, 89, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Ramsingh, A.I. CVB-induced pancreatitis and alterations in gene expression. Curr. Top. Microbiol. Immunol. 2008, 323, 241–258. [Google Scholar] [PubMed]
- Rhoades, R.E.; Tabor-Godwin, J.M.T.; Tsueng, G.; Feuer, R. Enterovirus infections of the central nervous system. Virol. J. 2011, 411, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Hammami, S.; el Mokni, R.; Faidi, K.; Falconieri, D.; Piras, A.; Procedda, S.; Mighri, Z.; el Aouni, M.H. Chemical composition and antioxidant activity of essential oil from aerial parts of Teucrium flavum L. subsp. flavum growing spontaneously in Tunisia. Nat. Prod. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, S.F.; Skanj, T.; Barrek, S.; Ghrabi, S.G.; Zarrouk, H. Composition of the essential oil of Teucrium ramosissimum Desf (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007, 22, 101–104. [Google Scholar] [CrossRef]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curimi, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.V.; Araújo, L.M.; de Oliveira, F.F.; da Conceiçäo, A.O.; Simoni, I.C.; Fernandes, M.J.B.; Weis, A. Avaliçӑo in vitro do potential antiviral de Guettarda angelica contra herpesvirus animais. Acta Sci. 2012, 40, 1–7. [Google Scholar]
- Ocazionez, R.E.; Menes, R.; Torres, F.À.; Stashenko, E. Virucidal activity of Colombian Lillia essential oils on dengue virus replication in vitro. Mem. Inst. Oswaldo Cruz 2010, 105, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K. Volatile composition and antimicrobial activity of the essential oil of Artemisia absinthium growing in Western Ghats region of North West Karnataka. Pharm. Biol. 2013, 51, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K. Chemical composition of the essential oil of Lepidagathis fasciculata from Bondla Forest of Goa. Nat. Prod. Commun. 2013, 8, 1163–1164. [Google Scholar] [PubMed]
- Joshi, R.K. Chemical constituents and antibacterial property of the essential oil of the roots of Cyathocline purpurea. J. Ethnopharmacol. 2013, 145, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Yu, L.; Zhao, M.; Wang, J.S.; Cui, C.; Yang, B.; Jiang, Y.; Zhao, Q. Antioxidant, immunomodulatory, andante-breast cancer activities of phenolic extract from pine (Pinus massoniana Lamb.) bark. Innov. Food Sci. Emerg. Technol. 2008, 9, 122–128. [Google Scholar] [CrossRef]
- Tian, W.; Lin, Q.; Liu, G.Q. In vitro antioxidant capacities of rice residue hydrolysates from fermented broth of five mold strains. J. Med. Plants Res. 2012, 6, 2396–2401. [Google Scholar]
- Armania, N.; Yazan, L.S.; Ismail, I.S.; Foo, J.B.; Tor, Y.S.; Ishak, N.; Ismail, N.; Ismail, M. Dillenia Suffruticosa extract inhibits proliferation of Human Breast Cancer cell lines (MCF-7 and MDA-MB-231) via induction of G2/M arrest and Apoptosis. Molecules 2013, 18, 13320–13339. [Google Scholar] [CrossRef] [PubMed]
- Ellithey, M.S.; Lall, N.; Hussein, A.A.; Meyer, D. Cytotoxic and HIV-1 enzyme inhibitory activities of Red sea marine organisms. BMC Complement. Altern. Med. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the essential oils are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammami, S.; Jmii, H.; Mokni, R.E.; Khmiri, A.; Faidi, K.; Dhaouadi, H.; Aouni, M.H.E.; Aouni, M.; Joshi, R.K. Essential Oil Composition, Antioxidant, Cytotoxic and Antiviral Activities of Teucrium pseudochamaepitys Growing Spontaneously in Tunisia. Molecules 2015, 20, 20426-20433. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules201119707
Hammami S, Jmii H, Mokni RE, Khmiri A, Faidi K, Dhaouadi H, Aouni MHE, Aouni M, Joshi RK. Essential Oil Composition, Antioxidant, Cytotoxic and Antiviral Activities of Teucrium pseudochamaepitys Growing Spontaneously in Tunisia. Molecules. 2015; 20(11):20426-20433. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules201119707
Chicago/Turabian StyleHammami, Saoussen, Habib Jmii, Ridha El Mokni, Abdelbaki Khmiri, Khaled Faidi, Hatem Dhaouadi, Mohamed Hédi El Aouni, Mahjoub Aouni, and Rajesh K. Joshi. 2015. "Essential Oil Composition, Antioxidant, Cytotoxic and Antiviral Activities of Teucrium pseudochamaepitys Growing Spontaneously in Tunisia" Molecules 20, no. 11: 20426-20433. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules201119707