Evaluation of the Cytotoxicity of Structurally Correlated p-Menthane Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antiproliferative Effect of Compounds
| Compounds | Cells | |||||
|---|---|---|---|---|---|---|
| HCT-116 | SD | OVCAR-8 | SD | SF-295 | SD | |
| IG% | IG% | IG% | ||||
| (−)-Carvone (1) | 11.94 | ±2.54 | 2.28 | ±1.38 | 12.28 | ±1.13 |
| (+)-Carvone (2) | 46.15 | ±2.46 | 48.07 | ±1.20 | 34.39 | ±3.47 |
| (−)-8-Hydroxycarvotanacetone (3) | 75.2 | ±2.62 | 94.01 | ±1.38 | 61.59 | ±3.10 |
| (+)-8-Hydroxycarvotanacetone (4) | 4.76 | ±1.85 | 3.12 | ±2.96 | 16.36 | ±1.07% |
| (−)-Carvone epoxide (5) | 29.24 | ±1.00 | 8.21 | ±0.49 | 10.93 | ±0.06 |
| (+)-Carveol epoxide (6) | 12.43 | ±4.31 | 4.58 | ±8.58 | 35.35 | ±2.44 |
| (−)- cis-Carveol (7) | 9 | ±2.38 | 3.61 | ±9.96 | 21.16 | ±1.19 |
| (−)-8-Acetoxycarvotanacetone (8) | 10.36 | ±9.38 | 1.62 | ±1.58 | 30.47 | ±3.51 |
| (+)-Pulegone (9) | 10.25 | ±5.85 | 14.41 | ±8.08 | 27.44 | ±9.95 |
| ( R)-Pulegone oxide (10) | 43.21 | ±2.31 | 17.62 | ±10.45 | 16.02 | ±6.43 |
| (−)- trans-Isopulegone (11) | 18.96 | ±5.08 | 5.98 | ±0.89 | 7.56 | ±6.73 |
| (+)-Limonene 1,2-epoxide (12) | 73.13 | ±2.77 | 93.1 | ±0.10 | 58.48 | ±1.07 |
| (−)-Sobrerol (13) | 9.78 | ±7.24 | 41.4 | ±4.20 | 34.21 | ±3.57 |
| ( S)-(−)-Perillyl alcohol (14) | 95.82 | ±0.30 | 91.68 | ±7.06 | 90.92 | ±0.39 |
| (−)-Perillaldehyde (15) | 83.03 | ±1.54 | 70.24 | ±1.43 | 59.28 | ±5.78 |
| (−)-Perillaldehyde 8,9-epoxide (16) | 98.64 | ±0.74 | 96.32 | ±1.51 | 99.89 | ±0.24 |
| (−)-Perillyl acetate (17) | 14.25 | ±5.38 | 3.06 | ±2.34 | 16.41 | ±4.32 |
| ( S)-Perillyl benzoate (18) | 5.02 | ±2.67 | 2.86 | ±1.72 | 4.53 | ±2.13 |
| Doxorubicin | 99.24 | ±0.15 | 100 | ±0.63 | 99.57 | ±0.31 |
2.2. Hemolytic Assay
| Cells | Doxorubicin µg/mL | (−)-Perillaldehyde 8,9-epoxide µg/mL | (−)-8-Hydroxycarvotanacetone µg/mL |
|---|---|---|---|
| HCT-116 | 0.01 0.01–0.02 | 1.03 0.79–1.34 | 1.08 0.71–1.42 |
| OVCAR-8 | 1.20 0.90–1.60 | 1.15 0.93–1.44 | 1.44 0.93–2.23 |
| SF-295 | 0.24 0.17–0.36 | 1.75 1.05–2.93 | 3.24 2.47–4.26 |
| HL-60 | 0.02 0.01–0.02 | 0.64 0.07–0.09 | ____ |
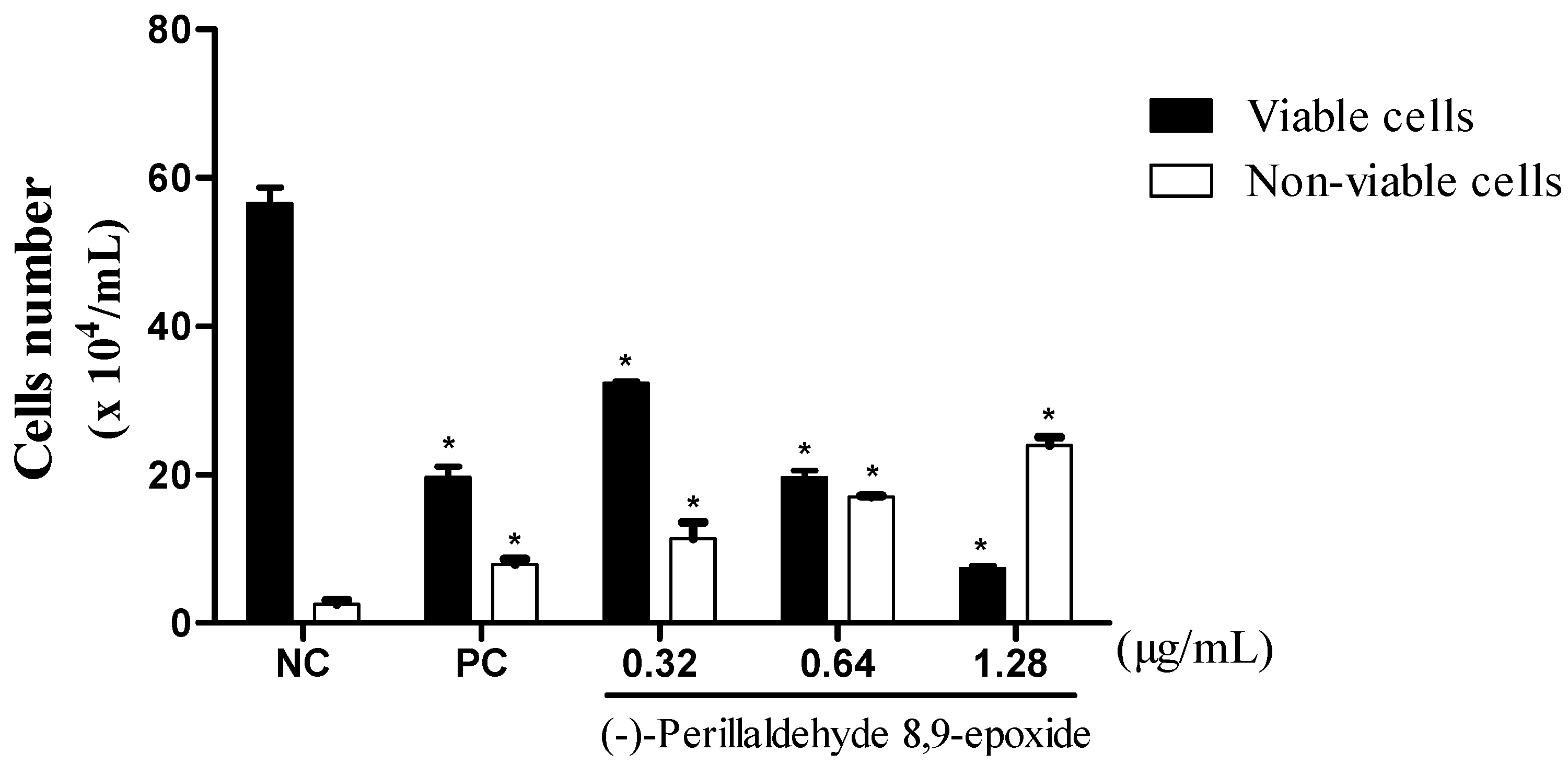
2.3. (−)-Perillaldehyde 8,9-epoxide Inhibits the Proliferation of Human Leukemia in HL-60 Cells
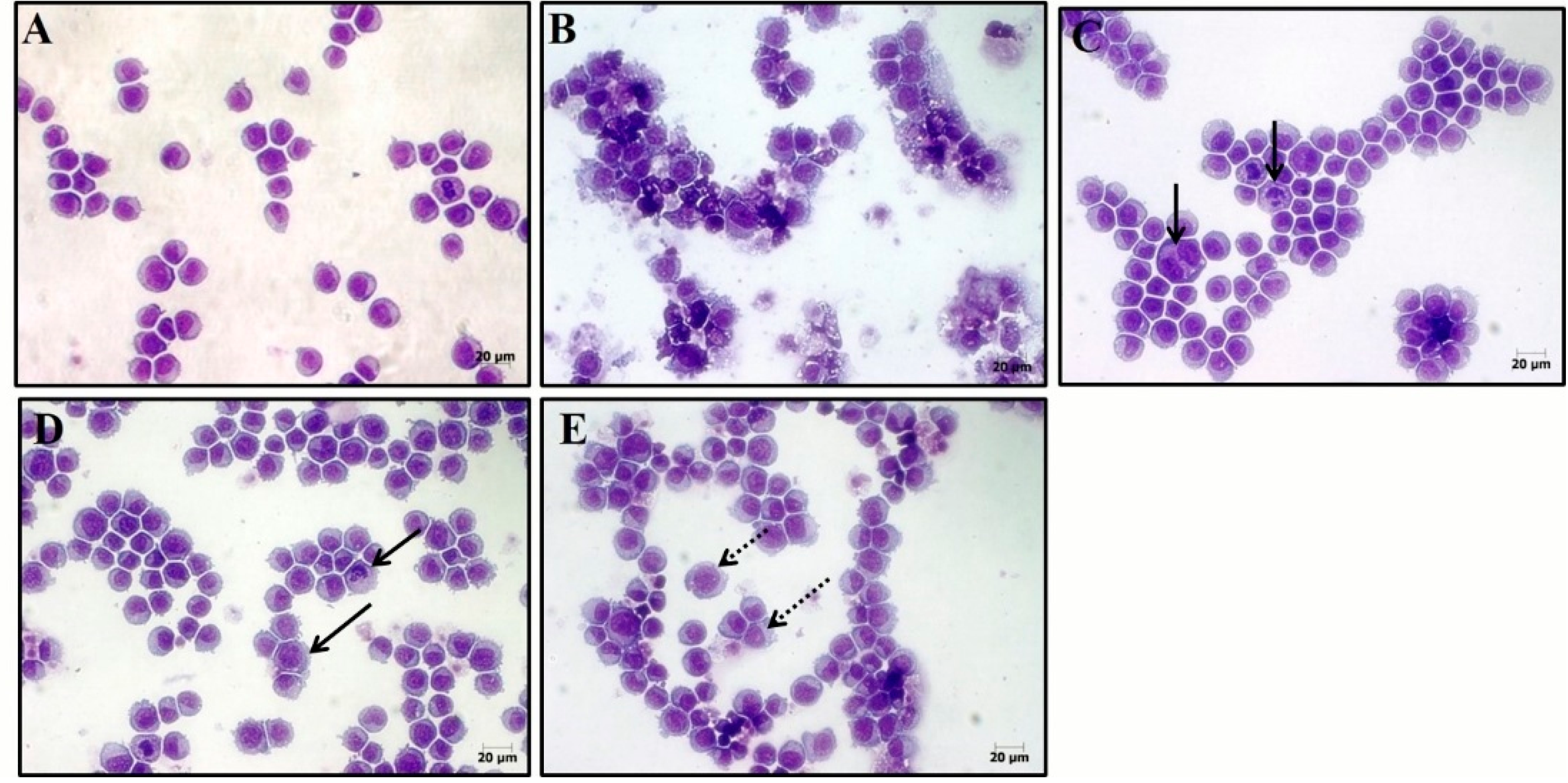
2.4. (−)-Perillaldehyde 8,9-epoxide Induces Apoptosis and Necrosis in Human Leukemia HL-60 Cells

2.5. Structure-Activity Relationships (SAR)
3. Experimental Section
3.1. Chemical Analogues
3.2. Cell Lines
3.3. Cytotoxicity Assay
3.4. Hemolytic Assay
3.5. Cell viability—Trypan Blue Dye Exclusion Test
3.6. Morphological Analyses Using a Fluorescence Microscope
3.7. Morphological Analysis with Hematoxylin-Eosin Staining
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.S.; de Jesus, A.M.; Dos Anjos, C.S.; da Silva, T.B.; Santos, A.D.C.; de Jesus, J.R.; Andrade, M.S.; Sampaio, T.S.; Gomes, W.F.; Alves, P.B.; et al. Evaluation of the cytotoxic activity of some Brasilian medicinal plants. Planta Med. 2012, 78, 1601–1606. [Google Scholar] [PubMed]
- Prakash, O.M.; Kumar, A.; Kumar, P.; Ajeet. Anticancer potential of plants and natural products: A review. Am. J. Pharmacol. Sci. 2013, 6, 104–115. [Google Scholar] [CrossRef]
- Ignacimuthu, S.; Ayyanar, M.; Sivaraman, S.K. Ethnobotanical investigations among tribes in Madurai district of Tamil Nadu (India). J. Ethnobiol. Ethnomed. 2006, 11, 2–25. [Google Scholar]
- Elujoba, A.A.; Odeleye, O.M.; Ogunyemi, C.M. Traditional medicine development for medical and dental primary health care delivery system in Africa. Afr. J. Tradit. Complement. Altern. Med. 2005, 2, 46–61. [Google Scholar] [CrossRef]
- Tomlinson, T.R.; Akerele, O. Medicinal Plants: Their Role in Health and Biodiversity; University of Pennsylvania Press: Philadelphia, PA, USA, 1998. [Google Scholar]
- Gordon, M.C.; David, J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar]
- Carvalho, A.A.; Andrade, L.N.; de Sousa, E.B.V.; de Sousa, D.P. Antitumor Phenylpropanoids Found in Essential Oils. Biomed. Res. Int. 2015, 1. [Google Scholar] [CrossRef]
- Su, Y.C.; Ho, C.L. Composition, in-vitro anticancer, and antimicrobial activities of the leaf essential oil of Machilus mushaensis from Taiwan. Nat. Prod. Commun. 2013, 8, 273–275. [Google Scholar] [PubMed]
- Manjamalai, A.; Grace, V.M.B. The chemotherapeutic effect of essential oil of Plectranthus amboinicus (Lour) on lung metastasis developed by B16F-10 cell line in C57BL/6 mice. Cancer Investig. 2013, 31, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Ashour, H.M. Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biol. Ther. 2008, 7, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.M.; Hashem, F.A.; Glombitza, K.W. Cytotoxicity and in vitro effects on human cancer cell lines of volatiles of Apium graveolens var. filicinum. Pharm. Pharmacol. Lett. 1998, 8, 97–99. [Google Scholar]
- Wattenberg, L.W. Inhibition of azoxymethane-induced neoplasia of the large bowel by 3-hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene (nerolidol). Carcinogenesis 1991, 12, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Leach, D.N.; Markham, J.K.; Markovic, B. In vitro cytotoxicity of Australian tea tree oil using human cell lines. J. Essent. Oil Res. 1997, 9, 575–582. [Google Scholar] [CrossRef]
- Manassero, C.A.; Girotti, J.R.; Mijailovsky, S.; Garcıa de Bravo, M.; Polo, M. In vitro comparative analysis of antiproliferative activity of essential oil from mandarin peel and its principal component limonene. Nat. Prod. Res. 2013, 2, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; de Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Belanger, J.T. Perillyl alcohol: Aplications in oncology. Altern. Med. Rev. 1998, 3, 448–457. [Google Scholar] [PubMed]
- Tan, G.; Gyllenhaal, C.; Soejarto, D.D. Biodiversity as a source of anticancer drugs. Curr. Drug. 2006, 7, 265–277. [Google Scholar] [CrossRef]
- Gould, M.N. Cancer chemoprevention and therapy by monoterpenes. Environ. Health Perspect. 1997, 105, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Gould, M.N. Induction of cytostasis in mammary carcinoma cells treated with the anticancer agent perillyl alcohol. Carcinogenesis 2002, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Pierre, K.J.; Elegbede, A.; Wang, R.C.; Carper, S.W. Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non small cell lung cancer cells. Cancer Lett. 2007, 257, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.J.; Burke, Y.D.; McKinzie, J.H.; Ayoubi, S.A.; Crowell, P.L. Chemotherapy of pancreatic cancer with the monoterpene perillyl alcohol. Cancer Lett. 1995, 96, 15–21. [Google Scholar] [CrossRef]
- Burke, Y.D.; Stark, M.J.; Roach, M.S.L.; Sen, S.E.; Crowell, P.L. Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids 1997, 32, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Balassiano, I.T.; Paulo, S.A.; Silva, N.H. Effects of perillyl alcohol in glial C6 cell line in vitro and anti-metastatic activity in chorioallantoic membrane. Int. J. Mol. Med. 2002, 10, 785–788. [Google Scholar]
- Elegbede, A.; Flores, R.; Wang, R.C. Perillyl alcohol and perillaldehyde induced cell cycle arrest and cell death in BroTo and A549 cells cultured in vitro. Life Sci. 2003, 73, 2831–2840. [Google Scholar] [CrossRef]
- Liu, G.; Oettel, K.; Bailey, H.H.; van Ummersen, L.; Tutsch, K.; Staab, M.J.; Horvath, D.; Alberti, D.; Arzoomanian, R.; Rezazadeh, H.; et al. Phase II trial of perillyl alcohol (NSC 641066) administered daily in patients with metastatic androgen independent prostate cancer. Investig. New Drugs 2003, 21, 367–372. [Google Scholar] [CrossRef]
- Bailey, H.H.; Attia, S.; Love, R.R.; Fass, T.; Chappell, R.; Tutsch, K.; Harris, L.; Jumonville, A.; Hansen, R.; Shapiro, G.R.; et al. Phase II trial of daily oral perillyl alcohol (NSC 641066) in treatment-refractory metastatic breast cancer. Cancer Chemother. Pharmacol. 2008, 62, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.L.; Paula, J.E.; Pessoa, C.; Moraes, M.O.; Costa-Lotufo, L.V.; Grougnet, R.; Michel, S.; Tillequin, F.; Espindola, L.S. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J. Ethnopharmacol. 2009, 123, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Santos-Júnior, H.M.; Oliveira, D.F.; Carvalho, D.A.; Pinto, J.M.A.; Campos, V.A.C.; Mourão, A.R.B.; Pessoa, C.; Moraes, M.O.; Costa-Lotufo, L.V. Evaluation of native and exotic Brazilian plants for anticancer activity. J. Nat. Med. 2010, 64, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, T.S.; Marques, M.R.; Pessoa, C.O.; Lotufo, L.V.C.; Magalhães, H.I.F.; Moraes, M.O.; Lima, D.P.; Tininis, A.G.; Oliveira, J.E. In vitro cytotoxic activity of Brazilian middle west plant extracts. Rev. Bras. Farmacogn. 2011, 21, 456–464. [Google Scholar] [CrossRef]
- Horvath, S. Cytotoxicity of drugs and diverse chemical agents to cell cultures. Toxicology 1980, 16, 59–66. [Google Scholar] [CrossRef]
- Karikas, G.A. Anticancer and chemopreventing natural products: Some biochemical and therapeutic aspects. J. B.U.ON. 2010, 15, 627–638. [Google Scholar]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Deb, D.D.; Parimala, G.; Devi, S.S.; Chakraborty, T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem. Biol. Int. 2011, 193, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.W.A.; Bandeira, P.N.; Lima, D.J.B.; Meira, A.S.; de Farias, S.S.; Albuquerque, M.R.J.R.; Dos Santos, H.S.; Lemos, T.L.G.; de Morais, M.O.; Costa-Lotufo, L.V. Amyrin esters induce cell death by apoptosis in HL-60 leukemia cells. Bioorg. Med. Chem. 2011, 19, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, H.I.F.; Wilke, D.V.; Bezerra, D.P.; Cavalcanti, B.C.; Rotta, R.; Lima, D.P.; Beatriz, A.; Moraes, M.O.; Diniz-Filho, J.; Pessoa, C. (4-Methoxyphenyl) (3,4,5-trimethoxyphenyl) methanone inhibits tubulin polimerization, induces G2/M arrest, and triggers apoptosis in human leucemia HL-60 cells. Toxicol. Appl. Pharmacol. 2013, 272, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, J.D. In vitro hemolysis of human erythrocytes by plant extracts with antiplasmodial activity. J. Ethnophamacol. 2001, 74, 239–243. [Google Scholar] [CrossRef]
- Aparicio, R.M.; Garcia-Celma, M.J.; Pilar, V.M.; Mitijans, M. In vitro studies of the hemolytic activity of microemulsions in human erythrocytes. J. Pharmaceut. Biomed. 2005, 39, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Lexis, L.A.; Fassett, R.G.; Coombes, J.S. Alpha-tocopherol and alpha-lipoic acid enhance the erythrocyte antioxidant defence in cyclosporine A-treated rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castañeda, J.R.; Montilla, P.; Padillo, F.J.; Bujalance, I.; Muñoz, M.C.; Muntané, J.; Tunez, I. Role of serotonin in cerebral oxidative stress in rats. Acta Neurobiol. Exp. 2006, 66, 1–6. [Google Scholar]
- Silva, S.L.; Chaar, J.S.; Figueiredo, P.M.S.; Yano, T. Cytotoxic evaluation of essential oil from Casearia sylvestris Sw on human cancer cells and erythrocytes. Acta Amazon. 2008, 38, 107–112. [Google Scholar] [CrossRef]
- Barros, F.W.; Bezerra, D.P.; Ferreira, P.M.; Cavalcanti, B.C.; Silva, T.G.; Pitta, M.G.; de Lima, M.C.; Galdino, S.L.; Pitta Ida, R.; Costa-Lotufo, L.V.; et al. Inhibition of DNA topoisomerase I activity and induction of apoptosis by thiazacridine derivatives. Toxicol. Appl. Pharmacol. 2013, 268, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Topham, C.H.; Taylor, S.S. Mitosis and apoptosis: How is the balance set? Curr. Opin. Cell Biol. 2013, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Mei, Y.; Sinha, S. Role of the crosstalk between autophagy and apoptosis in cancer. J. Oncol. 2013, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.M.; Edinger, A.L. The complex interplay between autophagy, apoptosis, and necrotic signals promotes T-cell homeostasis. Immunol. Rev. 2012, 236, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3488–3459. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Li, C.J.; Pinto, A.V.; Pardee, A.B. Release of mitochondrial cytochrome C in both apoptosis and necrosis induced by beta-lapachone in human carcinoma cells. Mol. Med. 1999, 5, 232–239. [Google Scholar] [PubMed]
- Zong, W.X.; Thompson, C.B. Necrotic death as a cell fate. Genes Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Katsuhara, J. Absolute configuration of pulegone oxide and piperitenone dioxide. J. Org. Chem. 1967, 32, 797–799. [Google Scholar] [CrossRef]
- Santos, R.B.T.; Brocksom, J.; Brocksom, U. A convenient deoxygenation of α,β-epoxy ketones to enones. Tetrahedron Lett. 1997, 38, 745–748. [Google Scholar] [CrossRef]
- Valeev, R.F.; Vostrikov, N.S.; Miftakhov, M.S. Synthesis and some transformations of (–)-carveol. Russian J. Org. Chem. 2009, 45, 810–814. [Google Scholar] [CrossRef]
- Lindquist, N.; Battiste, M.A.; Whitten, W.M.; Williams, N.H.; Strekowski, L. Trans-carvone Oxide, A Monoterpene Epoxide from the Fragrance of Catasetum. Phytochemistry 1985, 24, 863–865. [Google Scholar] [CrossRef]
- Thomas, A.F.; Bessière, Y. Limonene. Nat. Prod. Rep. 1989, 6, 291–309. [Google Scholar] [CrossRef]
- Halim, A.F.; Collins, R. P. Essential oil Salvia dorisiana (Standley). J. Agric. Food Chem. 1975, 23, 506–510. [Google Scholar] [CrossRef]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Text Book of Practical Organic Chemistry, 5th ed.; Addition Wesley Longman: London, UK, 1998. [Google Scholar]
- Kido, F.; Abiko, T.; Kato, M. Spiroannulation by the [2,3]sigmatropic rearrangement via the cyclic allylsulfonium ylide. A stereoselective synthesis of (+)-acorenone B. J. Chem. Soc. Perkin Trans. 1992, 2, 229–233. [Google Scholar] [CrossRef]
- Moreira, J.A.; Corrêa, A.G. Enantioselective synthesis of three stereoisomers of 5,9-dimethylpentadecane, sex pheromone component of Leucoptera coffeella, from (−)-isopulegol. Tetrahedron Asymmetry 2003, 14, 3787–3795. [Google Scholar] [CrossRef]
- Büchi, G.; Wuest, H.J. New synthesis of b-agarofuran and of dihydroagarofuran. J. Org. Chem. 1979, 44, 546–549. [Google Scholar] [CrossRef]
- Andrade, L.N.; Batista, J.S.; de Sousa, D.P. Spasmolytic activity of p-menthane esters. J. Med. Plant Res. 2011, 5, 6995–6999. [Google Scholar]
- Walling, C.T.; Lake, S.C.; Willis, U.C.R.; Mape Shade, N.J. Process for Preparing Cycloaliphatic Monoterpenic Alcohol. US Patent US4205184, 11 May 1976. [Google Scholar]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J. Methods in Plant Biochemistry: Assays for Bioactivity; Academic Press Inc.: London, UK, 1990. [Google Scholar]
- Jimenez, P.C.; Fortier, S.C.; Lotufo, T.M.C.; Pessoa, C.; Moraes, M.E.A.; Moraes, M.O.; Costa-Lotufo, L.V. Biological activity in extracts of ascidians (Tunicata, Ascidiacea) from the northeastern Brazilian coast. J. Exp. Mar. Biol. Ecol. 2003, 287, 93–101. [Google Scholar] [CrossRef]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.T.; Lee, H.; Kim, J.S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. 2009, 150, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Pita, J.C.L.R.; Xavier, A.L.; Sousa, T.K.G.; Mangueira, V.M.; Tavares, J.F.; Júnior, R.J.O.; Veras, R.C.; Pessoa, H.L.F.; Silva, M.S.; Morelli, S.; et al. In vitro and in vivo antitumor effect of trachylobane-360, a diterpene from Xylopia langsdorffiana. Molecules 2012, 17, 9573–9589. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Pessoa, C.; Moraes, M.O.J.; Silveira, E.R.; Lima, M.A.S.; Elmirob, F.J.M.; Costa-Lotufo, L.V. Antiproliferative effects of two amides, piperine and piplartine, from Piper species. Z. Naturforsch. C. 2005, 60, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Veras, M.L.; Bezerra, M.Z.; Braz-Filho, R.; Pessoa, O.D.; Montenegro, R.C.; Pessoa, C.; Moraes, M.O.; Costa-Lutufo, L.V. Cytotoxic epimeric withaphysalins from leaves of Acnistus arborescens. Planta Med. 2004, 70, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.X.; Zeng, Z.C.; Wang, J.Y. Docetaxel inhibits SMMC-7721 human hepatocellular carcinoma cells growth and induces apoptosis. World J. Gastroenterol. 2003, 9, 696–700. [Google Scholar] [PubMed]
- Sample Availability: Available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, L.N.; Lima, T.C.; Amaral, R.G.; Pessoa, C.D.Ó.; Filho, M.O.d.M.; Soares, B.M.; Nascimento, L.G.d.; Carvalho, A.A.; De Sousa, D.P. Evaluation of the Cytotoxicity of Structurally Correlated p-Menthane Derivatives. Molecules 2015, 20, 13264-13280. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules200713264
Andrade LN, Lima TC, Amaral RG, Pessoa CDÓ, Filho MOdM, Soares BM, Nascimento LGd, Carvalho AA, De Sousa DP. Evaluation of the Cytotoxicity of Structurally Correlated p-Menthane Derivatives. Molecules. 2015; 20(7):13264-13280. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules200713264
Chicago/Turabian StyleAndrade, Luciana Nalone, Tamires Cardoso Lima, Ricardo Guimarães Amaral, Cláudia Do Ó Pessoa, Manoel Odorico de Moraes Filho, Bruno Marques Soares, Lázaro Gomes do Nascimento, Adriana Andrade Carvalho, and Damião Pergentino De Sousa. 2015. "Evaluation of the Cytotoxicity of Structurally Correlated p-Menthane Derivatives" Molecules 20, no. 7: 13264-13280. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules200713264





