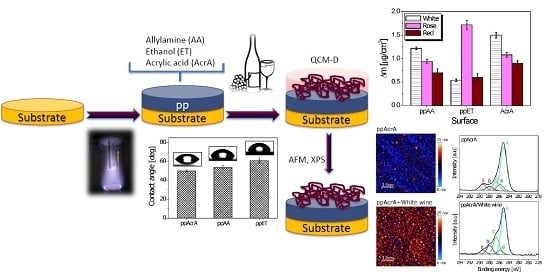
Adsorption of Wine Constituents on Functionalized Surfaces
Abstract
:1. Introduction
2. Results
2.1. NTA Measurements of Particle Size and Concentration
2.2. Surface Chemistry
2.2.1. Acrylic Acid Plasma Polymer (ppAcrA)
2.2.2. Allylamine Acid Plasma Polymer (ppAA)
2.2.3. Ethanol Acid Plasma Polymer (ppET)
2.3. Contact Angle Measurements
2.4. Quantification of Bound Wine
2.5. Atomic Force Microscopy
2.5.1. Topography
2.5.2. Water Content
3. Discussion
3.1. Adsorption on ppAA
3.2. Adsorption on ppAcrA
3.3. Adsorption on ppET
4. Materials and Methods
4.1. Wines
4.2. Chemicals
4.3. Plasma Polymerization
4.4. X-ray Photoelectron Spectroscopy (XPS)
4.5. Water Contact Angle
4.6. Quartz Crystal Microbalance with Dissipation (QCM-D)
4.7. Atomic Force Microscopy (AFM)
4.8. Nanoparticle Tracking Analysis (NTA)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| XPS | X-ray photoelectron spectroscopy |
| QCM-D | quartz crystal microbalance with dissipation monitoring |
| AFM | atomic force microscopy |
| NTA | nanoparticle tracking analysis |
| AA | allylamine |
| AcrA | acrylic acid |
| ET | ethanol |
| pp | plasma polymer |
| rms roughness | root mean square roughness |
| PTV | peak-to-valley-distance |
| ΔPTV | apparent layer thickness |
References
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, R.S.; Pais, A.; Linse, P.; Miguel, M.G.; Lindman, B. Polyion adsorption onto catanionic surfaces. A monte carlo study. J. Phys. Chem. B 2005, 109, 11781–11788. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Sakiyama, T.; Imamura, K. On the adsorption of proteins on solid surfaces, a common but very complicated phenomenon. J. Biosci. Bioeng. 2001, 91, 233–244. [Google Scholar] [CrossRef]
- Robertson, B.C.; Zydney, A.L. Protein adsorption in asymmetric ultrafiltration membranes with highly constricted pores. J. Colloid Interface Sci. 1990, 134, 563–575. [Google Scholar] [CrossRef]
- Lalande, M.; Tissier, J.P. Fouling of heat transfer surfaces related to β-lactoglobulin denaturation during heat processing of milk. Biotechnol. Prog. 1985, 1, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. Int. Ed. 2013, 52, 10766–10770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, K.; Ejima, H.; Yoshie, N. Antioxidant and adsorption properties of bioinspired phenolic polymers: A comparative study of catechol and gallol. ACS Sustain. Chem. Eng. 2016, 4, 3857–3863. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed]
- Pranantyo, D.; Xu, L.Q.; Neoh, K.G.; Kang, E.T.; Ng, Y.X.; Teo, S.L.M. Tea stains-inspired initiator primer for surface grafting of antifouling and antimicrobial polymer brush coatings. Biomacromolecules 2015, 16, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.S.; Hudson, D.M.; Loh, I.H. Biomaterial surface modification using plasma gas discharge processes. In Encyclopedic Handbook of Biomaterials and Bioengineering, Part A: Materials; Wise, D.L., Altobelli, D.E., Yaszemski, M.J., Gresser, J.D., Schwartz, E.R., Eds.; CRC Press, Taylor & Francis Group: New York, NY, USA, 1995; pp. 865–894. [Google Scholar]
- Mao, C.; Qiu, Y.Z.; Sang, H.B.; Mei, H.; Zhu, A.P.; Shen, J.; Lin, S.C. Various approaches to modify biomaterial surfaces for improving hemocompatibility. Adv. Colloid Interface Sci. 2004, 110, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.W.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.D.; Hlady, V. Protein adsorption and materials biocompatibility: A tutorial review and suggested hypotheses. Adv. Polym. Sci. 1986, 79, 1–63. [Google Scholar] [CrossRef]
- Agashe, M.; Raut, V.; Stuart, S.J.; Latour, R.A. Molecular simulation to characterize the adsorption behavior of a fibrinogen γ-chain fragment. Langmuir 2005, 21, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Martins, M.C.L.; Ratner, B.D.; Barbosa, M.A. Protein adsorption on mixtures of hydroxyl- and methyl-terminated alkanethiols self-assembled monolavers. J. Biomed. Mater. Res. A 2003, 67, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Tegoulia, V.A.; Rao, W.S.; Kalambur, A.T.; Rabolt, J.R.; Cooper, S.L. Surface properties, fibrinogen adsorption, and cellular interactions of a novel phosphorylcholine-containing self-assembled monolayer on gold. Langmuir 2001, 17, 4396–4404. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Smith, P.A. Current state of knowledge and challenges in wine clarification. Aust. J. Grape Wine Res. 2015, 21, 615–626. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Smith, P.A. Surface modification influencing adsorption of red wine constituents: The role of functional groups. Appl. Surf. Sci. 2016, 386, 14–23. [Google Scholar] [CrossRef]
- Yasuda, H. Plasma Polimerisation; Academic Press: Orlando, FL, USA, 1985. [Google Scholar]
- Yang, Z.L.; Wang, X.N.; Wang, J.; Yao, Y.; Sun, H.; Huang, N. Pulsed-plasma polymeric allylamine thin films. Plasma Proc. Polym. 2009, 6, 498–505. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Beamson, G.; Briggs, D. High. Resolution XPS of Organic Polymers: The Scienta Esca300 Handbook; Wiley: Chichester, UK, 1992. [Google Scholar]
- Fally, F.; Doneux, C.; Riga, J.; Verbist, J.J. Quantification of the functional-groups present at the surface of plasam polymers deposited from propylamine, allylamine, and propargylamine. J. Appl. Polym. Sci. 1995, 56, 597–614. [Google Scholar] [CrossRef]
- Liu, X.J.; Feng, Q.L.; Bachhuka, A.; Vasilev, K. Surface chemical functionalities affect the behavior of human adipose-derived stem cells in vitro. Appl. Surf. Sci. 2013, 270, 473–479. [Google Scholar] [CrossRef]
- Sinn, S.; Eichler, M.; Muller, L.; Bunger, D.; Groll, J.; Ziemer, G.; Rupp, F.; Northoff, H.; Geis-Gerstorfer, J.; Gehring, F.K.; et al. NCO-sP(EO-stat-PO) coatings on gold sensors—A QCM study of hemocompatibility. Sensors (Basel) 2011, 11, 5253–5269. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Perez, P.M.; Marques, A.P.; da Silva, R.M.P.; Pashkuleva, I.; Reis, R.L. Effect of chitosan membrane surface modification via plasma induced polymerization on the adhesion of osteoblast-like cells. J. Mater. Chem. 2007, 17, 4064–4071. [Google Scholar] [CrossRef] [Green Version]
- Hook, F.; Rodahl, M.; Brzezinski, P.; Kasemo, B. Measurements using the quartz crystal microbalance technique of ferritin monolayers on methyl-thiolated gold: Dependence of energy dissipation and saturation coverage on salt concentration. J. Colloid Interface Sci. 1998, 208, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Mierczynska-Vasilev, A.; Ralston, J.; Beattie, D.A. Adsorption of modified dextrins on talc: Effect of surface coverage and hydration water on hydrophobicity reduction. Langmuir 2008, 24, 6121–6127. [Google Scholar] [CrossRef] [PubMed]
- Hedin, J.; Lofroth, J.E.; Nyden, M. Adsorption behavior and cross-linking of ehec and HM-EHEC at hydrophilic and hydrophobic modified surfaces monitored by SPR and QCM-D. Langmuir 2007, 23, 6148–6155. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, J.; Agheli, H.; Kingshott, P.; Sutherland, D.S. Viscoelastic modeling of highly hydrated laminin layers at homogeneous and nanostructured surfaces: Quantification of protein layer properties using QCM-D and SPR. Langmuir 2007, 23, 9760–9768. [Google Scholar] [CrossRef] [PubMed]
- Hook, F.; Kasemo, B.; Nylander, T.; Fant, C.; Sott, K.; Elwing, H. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: A quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem. 2001, 73, 5796–5804. [Google Scholar] [CrossRef] [PubMed]
- Mierczynska, A.; Michelmore, A.; Tripathi, A.; Goreham, R.V.; Sedev, R.; Vasilev, K. pH-tunable gradients of wettability and surface potential. Soft Matter 2012, 8, 8399–8404. [Google Scholar] [CrossRef]
- Dufrechou, M.; Doco, T.; Poncet-Legrand, C.; Sauvage, F.X.; Vernhet, A. Protein/polysaccharide interactions and their impact on haze formation in white wines. J. Agric. Food Chem. 2015, 63, 10042–10053. [Google Scholar] [CrossRef] [PubMed]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—A review. Plasma Proc. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Van Sluyter, S.C.; Warnock, N.I.; Schmidt, S.; Anderson, P.; van Kan, J.A.; Bacic, A.; Waters, E.J. Aspartic acid protease from botrytis cinerea removes haze-forming proteins during white winemaking. J. Agric. Food Chem. 2013, 61, 9705–9711. [Google Scholar] [CrossRef] [PubMed]
- Whittle, J.D.; Barton, D.; Alexander, M.R.; Short, R.D. A method for the deposition of controllable chemical gradients. Chem. Commun. 2003, 1766–1767. [Google Scholar] [CrossRef]
- Robinson, D.E.; Marson, A.; Short, R.D.; Buttle, D.J.; Day, A.J.; Parry, K.L.; Wiles, M.; Highfield, P.; Mistry, A.; Whittle, J.D. Surface gradient of functional heparin. Adv. Mater. 2008, 20, 1166–1169. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds (wines, plasma polymers) are available from the authors.






| Sample | Γ (mg/m2) (QCM) | V × 10−8 (m−3) (QCM) 1 | V × 10−8 (m−3) (AFM) | Vw × 10−8 (m−3) Volume of Water (QCM) | % of Water |
|---|---|---|---|---|---|
| ppAcrA/White | 15.0 | 1.53 | 0.54 | 0.99 | 65 |
| ppAcrA/Rosé | 10.8 | 1.10 | 0.50 | 0.60 | 55 |
| ppAcrA/Red | 9.0 | 0.92 | 0.25 | 0.67 | 73 |
| ppAA/White | 12.2 | 1.24 | 0.20 | 1.04 | 84 |
| ppAA/Rosé | 9.4 | 0.96 | 0.18 | 0.78 | 81 |
| ppAA/Red | 7.0 | 0.71 | 0.22 | 0.49 | 69 |
| ppET/White | 5.4 | 0.55 | 0.06 | 0.49 | 89 |
| ppET/Rosé | 17.5 | 1.79 | 0.48 | 1.31 | 73 |
| ppET/Red | 6.0 | 0.61 | 0.16 | 0.45 | 74 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierczynska-Vasilev, A.; Smith, P.A. Adsorption of Wine Constituents on Functionalized Surfaces. Molecules 2016, 21, 1394. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21101394
Mierczynska-Vasilev A, Smith PA. Adsorption of Wine Constituents on Functionalized Surfaces. Molecules. 2016; 21(10):1394. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21101394
Chicago/Turabian StyleMierczynska-Vasilev, Agnieszka, and Paul A. Smith. 2016. "Adsorption of Wine Constituents on Functionalized Surfaces" Molecules 21, no. 10: 1394. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21101394