Chemistry and Pharmacology of Citrus sinensis
Abstract
:1. Introduction
2. Botanical Description
3. Traditional Uses
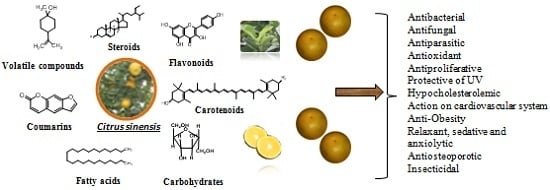
4. Chemical Composition
5. Pharmacological Activities
5.1. Antibacterial Activity
5.2. Antifungal Activity
5.3. Antiparasitic Activity
5.4. Antiproliferative Activity
5.5. Antioxidant Activity
5.6. Hypocholesterolemic Activity
5.7. Anti-Obesity Activity
5.8. Activity in Cardiovascular System
5.9. Antiosteoporotic Activity
5.10. Protective of UV Activity
5.11. Relaxant, Sedative and Anxiolytic Activities
5.12. Insecticidal Activity
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Moore, G.A. Oranges and lemons: Clues to the taxonomy of Citrus from molecular markers. Trends Genet. 2001, 17, 536–540. [Google Scholar] [CrossRef]
- Abbate, L.; Tusa, N.; del Bosco, S.F.; Strano, T.; Renda, A.; Ruberto, G. Genetic improvement of Citrus fruits: New somatic hybrids from Citrus sinensis (L.) Osb. and Citrus limon (L.) Burm F. Food Res. Int. 2012, 48, 284–290. [Google Scholar] [CrossRef]
- Barkley, N.A.; Roose, M.L.; Krueger, R.R.; Federici, C.T. Assessing genetic diversity and population structure in a citrus germplasm collection utilizing simple sequence repeat markers (SSRs). Theor. Appl. Genet. 2006, 112, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Cioni, P.L.; Morelli, I. Use of solid-phase micro-extraction as a sampling technique in the determination of volatiles emitted by flowers, isolated flower parts and pollen. J. Chromatogr. A 2003, 998, 229–233. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Intrigliolo, D.S.; Castel, J.R.; Fereres, E. Citrus. In Crop Yield response to Water: FAO Irrigation and Drainage Paper 66, 1st ed.; Pasquale Steduto, P., Theodore, C., Hsiao, T.C., Elias Fereres, E., Dirk Raes, D., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; Volume 49, pp. 300–315. [Google Scholar]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestree Database: A Tree Species Reference and Selection Guide Version 4.0; World Agroforestry Centre ICRAF: Nairobi, KE, USA, 2009; pp. 1–5. [Google Scholar]
- Han, S.T. Medicinal plants in the South Pacific, 1st ed.; World Health Organization (WHO) Regional Publications, Western Pacific Series: Manila, Philippines, 2008; pp. 49–50. [Google Scholar]
- Goudeau, D.; Uratsu, S.L.; Inoue, K.; daSilva, F.G.; Leslie, A.; Cook, D.; Reagan, L.; Dandekar, A.M. Tuning the orchestra: Selective gene regulation and orange fruit quality. Plant Sci. 2008, 174, 310–320. [Google Scholar] [CrossRef]
- Sharon-Asa, L.; Shalit, M.; Frydman, A.; Bar, E.; Holland, D.; Or, E.; Lavi, U.; Lewinsohn, E.; Eyal, Y. Citrus fruit flavor and aroma biosynthesis: Isolation, functional characterization, and developmental regulation of Cstps1, a key gene in the production of the sesquiterpene aroma compound valencene. Plant J. 2003, 36, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.N.; Soneji, J.R.; Sahijram, L. Citrus: Genoics Resources Developed. In Wild Crop Relatives: Genomic and Breeding Resources, 1st ed.; Chittaranjan, K., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2011; pp. 43–59. [Google Scholar]
- Ulloa, F.V.; García, S.S.; Estrada, M.C.; López, D.L.P.; Cruz, M.C.R.; García, P.S. Interpretation methods of nutrient diagnosis in orange cv. Valencia (Citrus sinensis L. Osbeck). Terra Latinoam. 2012, 30, 139–145. [Google Scholar]
- Etebu, E.; Nwauzoma, A.B. A review on sweet orange (Citrus Sinensis Osbeck): Health, diseases, and management. Am. J. Res. 2014, 2, 33–70. [Google Scholar]
- Milind, P.; Chaturvede, D. Orange: Range of benefits. Int. Res. J. Pharm. 2012, 3, 59–63. [Google Scholar]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of Citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, J.K.; Remsberg, C.M.; Yáñez, J.A.; Vega-Villa, K.R.; Davies, N.M. Stereospecific analysis of sakuranetin by high-performance liquid chromatography: Pharmacokinetic and botanical applications. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 875, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A. Fractionation of orange peel phenols in ultrafiltered molasses and mass balance studies of their antioxidant levels. J. Agric. Food Chem. 2004, 52, 7586–7592. [Google Scholar] [CrossRef] [PubMed]
- Rani, G.; Yadav, L.; Kalidhar, S.B. Chemical Examination of Citrus sinensis Flavedo Variety Pineapple. Indian J. Pharm. Sci. 2009, 71, 677–679. [Google Scholar] [PubMed]
- Intekhab, J.; Aslam, M. Isolation of a flavonoid from the roots of Citrus sinensis. Malays. J. Pharm. Sci 2009, 7, 1–8. [Google Scholar]
- Lapcík, O.; Klejdus, B.; Davidová, M.; Kokoska, L.; Kubán, V.; Moravcová, J. Isoflavonoids in the Rutaceae family: 1. Fortunella obovata, Murraya paniculata and four Citrus species. Phytochem. Anal. 2004, 15, 293–299. [Google Scholar]
- Saleem, M.; Farooq, A.; Ahmad, S.; Shafiq, N.; Riaz, N.; Jabbar, A.; Arshad, M.; Malik, A. Chemical constituents of Citrus sinensis var. Shukri from Pakistan. J. Asian Nat. Prod. Res. 2010, 12, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, U.; Caristi, C.; Panzera, V.; Licandro, G. Flavonoids in pigmented orange juice and second-pressure extracts. J. Agric. Food Chem. 2000, 48, 5501–5506. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; Ferreres, F.; Tomas-Barberan, F.A. Liquid chromatography-tandem mass spectrometry reveals the widespread occurrence of flavonoid glycosides in honey, and their potential as floral origin markers. J. Chromatogr. A 2009, 1216, 7241–7248. [Google Scholar] [CrossRef] [PubMed]
- Escudero-López, B.; Cerrillo, I.; Herrero-Martín, G.; Hornero-Méndez, D.; Gil-Izquierdo, A.; Medina, S.; Ferreres, F.; Berná, G.; Martín, F.; Fernández-Pachón, M.S. Fermented orange juice: Source of higher carotenoid and flavanone contents. J. Agric. Food Chem. 2013, 61, 8773–8782. [Google Scholar]
- Hillebrand, S.; Schwarz, M.; Winterhalter, P. Characterization of anthocyanins and pyranoanthocyanins from blood orange (Citrus sinensis (L.) Osbeck) juice. J. Agric. Food Chem. 2004, 52, 7331–7338. [Google Scholar] [CrossRef] [PubMed]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F.; Tomas-Barberan, F.A. In vitro availability of flavonoids and other phenolics in orange juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.J.; Beecher, G.R.; Bhagwat, S.A.; Dwyer, J.T.; Gebhardt, S.E.; Haytowitz, D. B.; Holden, J.M. Flavanones in grapefruit, lemons, and limes: A compilation and review of the data from the analytical literature. J. Food Comp. Anal. 2006, 19, S74–S80. [Google Scholar] [CrossRef]
- Stöggl, W.M.; Huck, C.W.; Stecher, G.; Bonn, G.K. Capillary electrochromatography of biologically relevant flavonoids. Electrophoresis 2006, 27, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lambros, T.; Wang, Z.; Goodnow, R.; Ho, C.T. Efficient and scalable method in isolation of polymethoxyflavones from orange peel extract by supercritical fluid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 846, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Yusa, T.; Sawabe, A.; Iizuka, Y.; Takekuma, S.; Yoshida, Y. Structures of new cyclic peptides in young unshiu (Citrus unshiu Marcov.), orange (Citrus sinensis Osbeck.) and amanatsu (Citrus natsudaidai) peelings. Agric. Biol. Chem. 1991, 55, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Kolhed, M.; Karlberg, B. Capillary electrophoretic separation of sugars in fruit juices using on-line mid infrared Fourier transform detection. Analyst 2005, 130, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Soler, C.; Hamilton, B.; Furey, A.; James, K.J.; Mañes, J.; Picó, Y. Comparison of four mass analyzers for determining carbosulfan and its metabolites in citrus by liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In Vitro Bioaccessibility of Carotenoids, Flavonoids, and Vitamin C from Differently Processed Oranges and Orange Juices (Citrus sinensis (L.) Osbeck). J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ariza, J.L.; García-Barrera, T.; Lorenzo, F. Determination of flavour and off-flavour compounds in orange juice by on-line coupling of a pervaporation unit to gas chromatography-mass spectrometry. J. Chromatogr. A 2004, 1047, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, H.; Tan, C.P.; Yusof, S.; Hamid, N.S. Solid-phase microextraction for determining twelve orange flavour compounds in a model beverage emulsion. Phytochem. Anal. 2008, 19, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Xie, B.J.; Zhang, Y.; Zhang, Y.; Fan, G.; Yao, X.L.; Pan, S.Y. Characterization of aroma active compounds in fruit juice and peel oil of Jinchen sweet orange fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O. Molecules 2008, 13, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cacho, P.R.; Mahattanatawee, K.; Smoot, J.M.; Rouseff, R. Identification of sulfur volatiles in canned orange juices lacking orange flavor. J. Agric. Food Chem. 2007, 55, 5761–5767. [Google Scholar] [CrossRef] [PubMed]
- Selli, S.; Canbas, A.; Varlet, V.; Kelebek, H.; Prost, C.; Serot, T. Characterization of the most odor-active volatiles of orange wine made from a Turkish cv. Kozan (Citrus sinensis L. Osbeck). J. Agric. Food Chem. 2008, 56, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.Y.; Hu, X.S.; Zhao, L.; Liao, X.J.; Wang, Z.F.; Wu, J.H. The characteristic analysis of several mineral contents in Chinese orange juice. Guang Pu Xue Yu Guang Pu Fen Xi 2009, 29, 259–262. (In Chinese) [Google Scholar] [PubMed]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Muthumary, J.; Srinivasan, K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Naila, A.; Nadia, D.; Zahoor, Q.S. Stable silver nanoparticles synthesis by Citrus sinensis (orange) and assessing activity against food poisoning microbes. Biomed. Environ. Sci. 2014, 27, 815–818. [Google Scholar] [PubMed]
- Chalova, V.I.; Crandall, P.G.; Ricke, S.C. Microbial inhibitory and radical scavenging activities of cold-pressed terpeneless Valencia orange (Citrus sinensis) oil in different dispersing agents. J. Sci. Food Agric. 2010, 90, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Muthaiyan, A.; Martin, E.M.; Natesan, S.; Crandall, P.G.; Wilkinson, B.J.; Ricke, S.C. Antimicrobial effect and mode of action of terpeneless cold-pressed Valencia orange essential oil on methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2012, 112, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Mayaud, L.; Carricajo, A.; Zhiri, A.; Aubert, G. Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Lett. Appl. Microbiol. 2008, 47, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and biological activities of decanal, linalool, valencene, and octanal from sweet orange oil. J. Food Sci. 2012, 77, C1156–C1161. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. In vitro inhibition of vancomycin-susceptible and vancomycin-resistant Enterococcus faecium and E. faecalis in the presence of citrus essential oils. Br. J. Biomed. Sci. 2009, 66, 180–185. [Google Scholar] [PubMed]
- Fisher, K.; Phillips, C. The mechanism of action of a citrus oil blend against Enterococcus faecium and Enterococcus faecalis. J. Appl. Microbiol. 2009, 106, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, C.A.; Crandall, P.G.; Chalova, V.I.; Ricke, S.C. Orange essential oils antimicrobial activities against Salmonella spp. J. Food Sci. 2008, 73, M264–M267. [Google Scholar] [CrossRef] [PubMed]
- Irkin, R.; Korukluoglu, M. Growth inhibition of pathogenic bacteria and some yeasts by selected essential oils and survival of L. monocytogenes and C. albicans in apple-carrot juice. Foodborne Pathog. Dis. 2009, 6, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Matiz, G.; Osorio, M.R.; Camacho, F.; Atencia, M.; Herazo, J. Effectiveness of antimicrobial formulations for acne based on orange (Citrus sinensis) and sweet basil (Ocimum basilicum L.) essential oils. Biomedica 2012, 32, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Corona, M.R.; Ramírez-Cabrera, M.A.; Santiago, O.G.; Garza-González, E.; Palacios, I.P.; Luna-Herrera, J. Activity against drug resistant-tuberculosis strains of plants used in Mexican traditional medicine to treat tuberculosis and other respiratory diseases. Phytother. Res. 2008, 22, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Guzeldag, G.; Kadioglu, L.; Mercimek, A.; Matyar, F. Preliminary examination of herbal extracts on the inhibition of Helicobacter pylori. Afr. J. Tradit. Complement. Altern. Med. 2013, 11, 93–96. [Google Scholar] [PubMed]
- Stange, R.R., Jr.; Midland, S.L.; Eckert, J.W.; Sims, J.J. An antifungal compound produced by grapefruit and Valencia orange after wounding of the peel. J. Nat. Prod. 1993, 56, 1627–1629. [Google Scholar] [CrossRef] [PubMed]
- Trovato, A.; Monforte, M.T.; Forestieri, A.M.; Pizzimenti, F. In vitro anti-mycotic activity of some medicinal plants containing flavonoids. Boll. Chim. Farm. 2000, 139, 225–227. [Google Scholar] [PubMed]
- Singh, P.; Shukla, R.; Prakash, B.; Kumar, A.; Singh, S.; Mishra, P.K.; Dubey, N.K. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, dl-limonene. Food Chem. Toxicol. 2010, 48, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, X.; Cheng, D.; Yao, X.; Pan, S. Structure-activity relationship of citrus polymethoxylated flavones and their inhibitory effects on Aspergillus niger. J. Agric. Food Chem. 2012, 60, 4336–4341. [Google Scholar] [CrossRef] [PubMed]
- Van-Hung, P.; Chi, P.T.; Phi, N.T. Comparison of antifungal activities of Vietnamese citrus essential oils. Nat. Prod. Res. 2013, 27, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Bagavan, A.; Rahuman, A.A.; Kamaraj, C.; Kaushik, N.K.; Mohanakrishnan, D.; Sahal, D. Antiplasmodial activity of botanical extracts against Plasmodium falciparum. Parasitol Res 2011, 108, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.P.; Surolia, N. In vitro antimalarial activity of extracts of three plants used in the traditional medicine of India. Am. J. Trop. Med. Hyg. 2001, 65, 304–308. [Google Scholar] [PubMed]
- Habila, N.; Agbaji, A.S.; Ladan, Z.; Bello, I.A.; Haruna, E.; Dakare, M.A.; Atolagbe, T.O. Evaluation of In Vitro Activity of Essential Oils against Trypanosoma brucei brucei and Trypanosoma evansi. J. Parasitol. Res. 2010, 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Vitali, F.; Pennisi, C.; Tomaino, A.; Bonina, F.; De Pasquale, A.; Saija, A.; Tita, B. Effect of a standardized extract of red orange juice on proliferation of human prostate cells in vitro. Fitoterapia 2006, 77, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Camarda, L.; di Stefano, V.; del Bosco, S.F.; Schillaci, D. Antiproliferative activity of Citrus juices and HPLC evaluation of their flavonoid composition. Fitoterapia 2007, 78, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Chinedu, E.; Arome, D.; Ameh, S.F.; Ameh, G.E. Evaluation of the anti-proliferative and cytostatic effect of Citrus sinensis (orange) fruit juice. Int. J. Appl. Basic Med. Res. 2014, 4 (Suppl. S1), S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Yang, C.S.; Li, S.; Jin, H.; Ho, C.T.; Patel, T. Monodemethylated polymethoxyflavones from sweet orange (Citrus sinensis) peel inhibit growth of human lung cancer cells by apoptosis. Mol. Nutr. Food Res. 2009, 53, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N.; Ho, C.T.; Li, S.; Colby, J.; Dushenkov, S. Apoptosis-inducing activity of hydroxylated polymethoxyflavones and polymethoxyflavones from orange peel in human breast cancer cells. Mol. Nutr. Food Res. 2007, 51, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Kurihara, N.; Abe, S.; Ho, C.T.; Ghai, G.; Yang, K. Chemopreventive effects of orange peel extract (OPE). I: OPE inhibits intestinal tumor growth in ApcMin/+ mice. J. Med. Food 2007, 10, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pan, M.H.; Lai, C.S.; Lo, C.Y.; Dushenkov, S.; Ho, C.T. Isolation and syntheses of polymethoxyflavones and hydroxylated polymethoxyflavones as inhibitors of HL-60 cell lines. Bioorg. Med. Chem. 2007, 15, 3381–3389. [Google Scholar] [CrossRef] [PubMed]
- Chidambara-Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. d-limonene rich volatile oil from blood oranges inhibits angiogenesis, metastasis and cell death in human colon cancer cells. Life Sci. 2012, 91, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N.; Li, S.; Colby, J.; Ho, C.T.; Dushenkov, S. Polymethoxylated flavones induce Ca(2+)-mediated apoptosis in breast cancer cells. Life Sci. 2006, 80, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Trovato, A.; Monforte, M.T.; Rossitto, A.; Forestieri, A.M. In vitro cytotoxic effect of some medicinal plants containing flavonoids. Boll. Chim. Farm. 1996, 135, 263–266. [Google Scholar] [PubMed]
- Genovese, S.; Fiorito, S.; Locatelli, M.; Carlucci, G.; Epifano, F. Analysis of biologically active oxyprenylated ferulic acid derivatives in Citrus fruits. Plant Foods Hum. Nutr. 2014, 69, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Karyakina, E.E.; Vokhmyanina, D.V.; Sizova, N.V.; Sabitov, A.N.; Borisova, A.V.; Sazontova, T.G.; Arkhipenko, Y.V.; Tkachuk, V.A.; Zolotov, Y.A.; Karyakin, A.A. Kinetic approach for evaluation of total antioxidant activity. Talanta 2009, 80, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First evidence of C- and O glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties. Food Chem. 2014, 149, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Cortina-Puig, M.; Muñoz-Berbel, X.; Rouillon, R.; Calas-Blanchard, C.; Marty, J.L. Development of a cytochrome c-based screen-printed biosensor for the determination of the antioxidant capacity of orange juices. Bioelectrochemistry 2009, 76, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, O.M. The antioxidant activity and polyphenolic contents of different plant seeds extracts. Pak. J. Biol. Sci. 2009, 12, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Kanaze, F.I.; Termentzi, A.; Gabrieli, C.; Niopas, I.; Georgarakis, M.; Kokkalou, E. The phytochemical analysis and antioxidant activity assessment of orange peel (Citrus sinensis) cultivated in Greece-Crete indicates a new commercial source of hesperidin. Biomed. Chromatogr. 2009, 23, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Uddin, G.; Ali, J. Phytochemical analysis and radical scavenging profile of juices of Citrus sinensis, Citrus anrantifolia, and Citrus limonum. Org. Med. Chem. Lett. 2014, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O. Characterization of the antioxidant properties of phenolic extracts from some citrus peels. J. Food Sci. Technol. 2012, 49, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Hrelia, S.; Angeloni, C.; Morroni, F.; Biagi, P.; Guardigli, M.; Cantelli-Forti, G.; Hrelia, P. Antioxidant effectiveness of organically and non-organically grown red oranges in cell culture systems. Eur. J. Nutr. 2006, 45, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, B.; Dar, K.K.; Ali, S.; Awan, U.A.; Nayyer, A.Q.; Ghous, T.; Andleeb, S. Short Communication: In vitro assessment of antioxidant, antibacterial and phytochemical analysis of peel of Citrus sinensis. Pak. J. Pharm. Sci. 2015, 28, 231–239. [Google Scholar] [PubMed]
- Tounsi, M.S.; Wannes, W.A.; Ouerghemmi, I.; Jegham, S.; Ben-Njima, Y.; Hamdaoui, G.; Zemni, H.; Marzouk, B. Juice components and antioxidant capacity of four Tunisian Citrus varieties. J. Sci. Food Agric. 2011, 91, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Kokkalou, E.; Assimopoulou, A.N.; Papageorgiou, V.P. Analysis of antioxidant compounds in sweet orange peel by HPLC-diode array detection-electrospray ionization mass spectrometry. Biomed. Chromatogr. 2005, 19, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Trovato, A.; Monforte, M.T.; Barbera, R.; Rossitto, A.; Galati, E.M.; Forestieri, A.M. Effects of fruit juices of Citrus sinensis L. and Citrus limon L. on experimental hypercholesterolemia in the rat. Phytomedicine 1996, 2, 221–227. [Google Scholar] [CrossRef]
- Wu, S.C.; Wu, S.H.; Chau, C.F. Improvement of the hypocholesterolemic activities of two common fruit fibers by micronization processing. J. Agric. Food Chem. 2009, 57, 5610–5614. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Graziano, A.C.; Venditti, A. Clinical evaluation of Moro (Citrus sinensis (L.) Osbeck) orange juice supplementation for the weight management. Nat. Prod. Res. 2015, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xi, W.; Ding, X.; Fan, S.; Zhang, Y.; Jiang, D.; Li, Y.; Huang, C.; Zhou, Z. Citrange Fruit Extracts Alleviate Obesity-Associated Metabolic Disorder in High-Fat Diet-Induced Obese C57BL/6 Mouse. Int. J. Mol. Sci. 2013, 14, 23736–23750. [Google Scholar] [CrossRef] [PubMed]
- Ramful, D.; Tarnus, E.; Rondeau, P.; Robert -Da Silva, C.; Bahorun, T.; Bourdon, E. Citrus Fruit Extracts Reduce Advanced Glycation End Products (AGEs)- and H2O2-Induced Oxidative Stress in Human Adipocytes. J. Agric. Food Chem. 2010, 58, 11119–11129. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Keshvari, M. Effects of Citrus sinensis juice on blood pressure. ARYA Atheroscler 2013, 9, 98–101. [Google Scholar] [PubMed]
- Oliveira, E.D.; Leite, T.S.; Silva, B.A.; Conde-Garcia, E.A. Inotropic effect of Citrus sinensis (L.) Osbeck leaf extracts on the guinea pig atrium. Braz. J. Med. Biol. Res. 2005, 38, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, N.M.M.; Abd-Alla, H.I.; Ahmed, H.H.; Basoudan, N. Protective effect of Citrus sinensis and Citrus aurantifolia against osteoporosis and their phytochemical constituents. J. Med. Plants Res. 2011, 5, 579–588. [Google Scholar]
- Morrow, R.; Deyhim, F.; Patil, B.S.; Stoecker, B.J. Feeding orange pulp improved bone quality in a rat model of male osteoporosis. J. Med. Food 2009, 12, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Cimino, F.; Cristani, M.; Saija, A.; Bonina, F.P.; Virgili, F. Protective effects of a red orange extract on UVB-induced damage in human keratinocytes. Biofactors 2007, 30, 129–238. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Offerta, A.; Saija, A.; Trombetta, D.; Venera, C. Protective effect of red orange extract supplementation against UV-induced skin damages: Photoaging and solar lentigines. J. Cosmet. Dermatol. 2014, 13, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lehrner, J.; Eckersberger, C.; Walla, P.; Pötsch, G.; Deecke, L. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol. Behav. 2000, 71, 83–86. [Google Scholar] [CrossRef]
- Díaz-Juárez, J.A.; Tenorio-López, F.A.; Zarco-Olvera, G.; Valle-Mondragón, L.D.; Torres-Narváez, J.C.; Pastelín-Hernández, G. Effect of Citrus paradisi extract and juice on arterial pressure both in vitro and in vivo. Phytother. Res. 2009, 23, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Goes, T.C.; Antunes, F.D.; Alves, P.B.; Teixeira-Silva, F. Effect of sweet orange aroma on experimental anxiety in humans. J. Altern. Complement. Med. 2012, 18, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Faturi, C.B.; Leite, J.R.; Alves, P.B.; Canton, A.C.; Teixeira-Silva, F. Anxiolytic-like effect of sweet orange aroma in Wistar rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Traboulsi, A.F.; El-Haj, S.; Tueni, M.; Taoubi, K.; Nader, N.A.; Mrad, A. Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Manag. Sci. 2005, 61, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.M.; Bertoni, A.; Rossi, Y.; Santander, R.; Urzúa, A. Efficacy of essential oils from edible plants as insecticides against the house fly, Musca domestica L. Molecules 2009, 14, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Rossi, Y.E.; Palacios, S.M. Fumigant toxicity of Citrus sinensis essential oil on Musca domestica L. adults in the absence and presence of a P450 inhibitor. Acta Trop. 2013, 127, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal evaluation of essential oils of Citrus sinensis L. (Myrtales: Myrtaceae) against housefly, Musca domestica L. (Diptera: Muscidae). Parasitol. Res. 2012, 110, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Hussain, D.; Rashid, R.H.; Salem, H.M.; Ghouse, G.; Abbas, M. Insecticidal activities of two citrus oils against Tribolium castaneum (herbst). N. Y. Sci. J. 2013, 6, 17–20. [Google Scholar]
- Ezeonu, F.C.; Chidume, G.I.; Udedi, S.C. Insecticidal properties of volatile extracts of orange peels. Bioresour. Technol. 2001, 76, 273–274. [Google Scholar] [CrossRef]
- Karamaouna, F.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.; Polissiou, M.; Papatsakona, P.; Tsora, E. Insecticidal activity of plant essential oils against the vine mealybug, Planococcus ficus. J. Insect Sci. 2013, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moravvej, G.; Abbar, S. Fumigant toxicity of citrus oils against cowpea seed beetle Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Pak. J. Biol. Sci. 2008, 11, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bagavan, A.; Kamaraj, C.; Rahuman, A.A.; Elango, G.; Zahir, A.A.; Pandiyan, G. Evaluation of larvicidal and nymphicidal potential of plant extracts against Anopheles subpictus Grassi, Culex tritaeniorhynchus Giles and Aphis gossypii Glover. Parasitol. Res. 2009, 104, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Mahesh-Kumar, P.; Kovendan, K.; Amerasan, D.; Subrmaniam, J.; Hwang, J.S. Larvicidal, pupicidal, repellent and adulticidal activity of Citrus sinensis orange peel extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2012, 111, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Mwaiko, G.L. Citrus peel oil extracts as mosquito larvae insecticides. East Afr. Med. J. 1992, 69, 223–226. [Google Scholar] [PubMed]
- Phasomkusolsil, S.; Soonwera, M. Efficacy of herbal essential oils as insecticide against Aedes aegypti (Linn.), Culex quinquefasciatus (Say) and Anopheles dirus (Peyton and Harrison). Southeast Asian J. Trop. Med. Public Health 2011, 42, 1083–1092. [Google Scholar] [PubMed]
- Araújo, C.P., Jr.; da Camara, C.A.; Neves, I.A.; Ribeiro, N.C.; Gomes, C.A.; de Moraes, M.M.; Botelho, P.S. Acaricidal activity against Tetranychus urticae and chemical composition of peel essential oils of three Citrus species cultivated in NE Brazil. Nat. Prod. Commun. 2010, 5, 471–476. [Google Scholar] [PubMed]
- Ribeiro, N.C.; da Camara, C.A.; Born, F.S.; de Siqueira, H.A. Insecticidal activity against Bemisia tabaci biotype B of peel essential oil of Citrus sinensis var. pear and Citrus aurantium cultivated in northeast Brazil. Nat. Prod. Commun. 2010, 5, 1819–1822. [Google Scholar]
- Salwa, M.H.; Abdel-Shafy, S.; Youssef, A.G. Light, scanning electron microscopy and SDS-PAGE studies on the effect of the essential oil, Citrus sinensis var. balady on the embryonic development of camel tick Hyalomma dromedarii (Koch, 1818) (Acari: Ixodidae). Pak. J. Biol. Sci. 2007, 10, 1151–1160. [Google Scholar] [PubMed]
- Warikoo, R.; Ray, A.; Sandhu, J.K.; Samal, R.; Wahab, N.; Kumar, S. Larvicidal and irritant activities of hexane leaf extracts of Citrus sinensis against dengue vector Aedes aegypti L. Asian Pac. J. Trop. Biomed. 2012, 2, 152–155. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Abdelnur, P.V.; Garcia, C.F.; Belini, A.; Severino, V.G.; da Silva, M.F.; Fernandes, J.B.; Vieira, P.C.; de Carvalho, S.A.; de Souza, A.A.; et al. Chemical characterization of Citrus sinensis grafted on C. limonia and the effect of some isolated compounds on the growth of Xylella fastidiosa. J. Agric. Food Chem. 2008, 56, 7815–7822. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ding, Z.; Yang, L.; Xu, L.; Zhang, G.; Li, K. A preliminary study on bioactivity of orange and tangerine peel extracts against aphis and mites. Zhongguo Zhong Yao Za Zhi 1995, 20, 397–398. (In Chinese) [Google Scholar] [PubMed]
- Zhou, X.; Shen, Z.; Gong, Q.; Zhu, C.; Gao, J.; Yue, J. Research progress on processing and comprehensive utilization of sweet orange. Zhongguo Niangzao 2015, 34, 13–17. [Google Scholar]
- Rani, G.; Kalidhar, S.B. Chemical constituents of Citrus sinensis varietis: A review. J. Med. Aromat. Plant Sci. 2010, 32, 324–350. [Google Scholar]
- Di Giacomo, A. Essential oils of citrus fruits. Essential oils of sweet orange. Riv. Ital. Essenze Profum. Piante Off. Aromi Sapon. Cosmet. Aerosol 1973, 55, 763–773. [Google Scholar]














| Compound | Region of Collection | Plant Organ | References |
|---|---|---|---|
| Flavonoids: 1–54 | United States: Washington, Florida (2, 3, 21, 27, 50–54). India: Hisar, Shahjahanpur (1, 4). Pakistan (19, 28). Italy: Sicily, Messina (17, 18, 20, 22–26, 29–34). Spain: Murcia, Huelva (5–16). Germany: Braunschweig (35–42). Czech Republic: Prague (43–49) | Peel, flavedo, molasses, whole fruit, leaves | [15,16,17,18,19,20,21,22,23,24,25] |
| Steroids: 55–56 | United States: Washington (55–56) | Leaves | [18] |
| Hydroxylamide, alkane, Fatty acids: 57–60 | United States: Washington (57–60) | Leaves | [18] |
| Coumarins: 61–67 | India: Shahjahanpur (61) United States: Florida (Lakeland) (62–67) | Peel, root | [26,27,28,29] |
| Peptides: 68–70 | Japan: Wakayama (68–70) | Peel | [30] |
| Carbohydrates: 71–74 | Sweden: Stockholm (71–74) | Fruit | [31] |
| Carbamates, alkylamines: 75–78 | Spain: Valencia (75–78) | Fruit | [32] |
| Carotenoids: 79–82 | Germany: Stuttgart (79–82) | Fruit | [33] |
| Volatile compounds: 83–148 | Spain: Huelva (83–85, 87, 88, 90, 121–124, 138–141). China: Songzi (Hubei) (137, 125, 126, 143–148). Turkey: Dortyol–Hatay, Kozan (98–101, 105–110, 116, 120). United States: Florida (92–97, 111–119, 127–131). Germany: Steinheim (86, 89, 91, 102–104, 132–136, 142) | Fruit, orange blossom, peel, leave | [34,35,36,37,38,39] |
| Potassium, magnesium, calcium and sodium | China: Beijing | Natural and commercial juices | [40] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favela-Hernández, J.M.J.; González-Santiago, O.; Ramírez-Cabrera, M.A.; Esquivel-Ferriño, P.C.; Camacho-Corona, M.D.R. Chemistry and Pharmacology of Citrus sinensis. Molecules 2016, 21, 247. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21020247
Favela-Hernández JMJ, González-Santiago O, Ramírez-Cabrera MA, Esquivel-Ferriño PC, Camacho-Corona MDR. Chemistry and Pharmacology of Citrus sinensis. Molecules. 2016; 21(2):247. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21020247
Chicago/Turabian StyleFavela-Hernández, Juan Manuel J., Omar González-Santiago, Mónica A. Ramírez-Cabrera, Patricia C. Esquivel-Ferriño, and María Del Rayo Camacho-Corona. 2016. "Chemistry and Pharmacology of Citrus sinensis" Molecules 21, no. 2: 247. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21020247