Synthesis and Properties of MQ Copolymers: Current State of Knowledge
Abstract
:1. Introduction
2. Results
2.1. Obtaining of MQ Copolymers by Hydrolytic Polycondensation
2.2. Obtaining of MQ Copolymers by Heterofunctional Polycondensation
2.3. Obtaining of MQ Copolymers Based on Silicic Acids and Silicates
2.4. Obtaining of MQ-Copolymers by Polycondensation in Active Medium
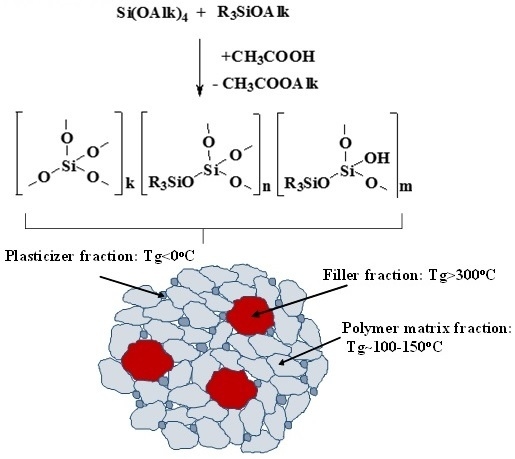
2.5. Structure and Properties of MQ Copolymers
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Norton, F.J. Production of Water-Repellent Materials. U.S. Patent 2,412,470, 10 December 1946. [Google Scholar]
- Goodwin, J.J.T. Organopolysiloxane Compositions Having Pressure-Sensitive Adhesive Properties. U.S. Patent 2,857,356, 21 October 1958. [Google Scholar]
- Lamoreaux, H.F.; Modic, F.J. Tough Unsupported Films Formed from Organopolysiloxanes. U.S. Patent 3,629,358, 21 December 1971. [Google Scholar]
- Modic, F.J. Silicone Potting Compositions Comprising Mixtures of Organopolysiloxanes Containing Vinyl Groups. U.S. Patent 3,436,366, 1 April 1969. [Google Scholar]
- James, F.H. Organo-Siloxanes and Methods of Making Them. U.S. Patent 2,441,320, 11 May 1948. [Google Scholar]
- Colas, A.R.L.; Geilich, K.M. Elastomer-Forming Compositions Comprising Alkoxylated MQ Resins and Polydiorganosiloxanes. U.S. Patent 5,091,484, 25 February 1992. [Google Scholar]
- Mbah, G.C. Optically Clear Reinforced Organosiloxane Compositions. U.S. Patent 4,882,398, 21 November 1989. [Google Scholar]
- Gordan, G.V.; Schmidt, R.G.; Stark-Kasley, L.A.; Wieber, G.M. MQ and T-Propyl Siloxane Resins Compositions. U.S. Patent 7,803,358, 28 September 2010. [Google Scholar]
- Gould, G.B.; Mitchell, T.P. Organosiloxane Compositions and Coatings, Manufactured Articles, Methods and Uses. U.S. Patent 2016/0,053,056, 25 February 2016. [Google Scholar]
- Mine, K.; Maruyama, T.; Takeshita, K. Siloxane Compositions which Form Ceramics at High Temperatures. U.S. Patent 4,269,757, 26 May 1981. [Google Scholar]
- Shirahata, A. Method for Producing Organosilicon Polymers and the Polymers Prepared Thereby. U.S. Patent 470,753, 17 November 1987. [Google Scholar]
- Tsumura, H.; Mutoh, K.; Satoh, K.; Isobe, K. Method for the Preparation of an Organopolysiloxane Containing Tetrafunctional Siloxane Units. U.S. Patent 5,070,175, 3 December 1991. [Google Scholar]
- Herzig, C.; Zoellner, O.; Hockemeyer, F.; Banfic, R. Cross-Linkable Compounds, Optionally Containing MQ Silicon Resins. U.S. Patent 6,274,692, 14 August 2001. [Google Scholar]
- Rust, J.B. Organo-Silicon Copolymers and Process of Making Same. U.S. Patent 2,562,953, 7 August 1951. [Google Scholar]
- Beger, A.; Lower, L.; Lueder, T.; Nesbitt, R.; Schmidt, R. Pressure Sensitive Adhesives and Methods for Their Preparation. U.S. Patent 8,298,367, 30 October 2012. [Google Scholar]
- Serobian, A.K. Aqueous Composition and Method for Imparting Resistance to Stain Absorption. U.S. Patent 7,645,333, 12 January 2010. [Google Scholar]
- Araud, C. Polydimethylsiloxane/MQ Resin Antifoaming Compositions. U.S. Patent 5,082,590, 21 January 1992. [Google Scholar]
- Guillaume, K.; Xavier, T.; Garaud, J.-L. Cosmetic Process For Coating Keratin Material. W.O. Patent 2011001220, 6 January 2011. [Google Scholar]
- Magee, W.L.; Emerson, A.W.; Joslyn, W.G.; Odneal, R.S. MQ Resins from Stable Ethylsilicate Polymer. U.S. Patent 8,829,144, 9 September 2014. [Google Scholar]
- Cho, H.J.; Kim, K.N.; Choi, K.H.; Choi, Y.J. Make-Up Cosmetic Composition Containing Mq Silicone Resin and Propyl Silsesquioxane Resin. U.S. Patent 2016/0,374,929, 29 December 2016. [Google Scholar]
- Lewis, L.N.; Wengrovius, J.H.; Burnell, T.B.; Rich, J.D. Powdered MQ Resin—Platinum Complexes and Their Use as Silicone-Soluble Hydrosilylation Cure Catalysts. Chem. Mater. 1997, 9, 761–765. [Google Scholar] [CrossRef]
- Di, M.; He, S.; Li, R.; Yang, D. Radiation effect of 150 keV protons on methyl silicon rabber reinforced with MQ silicone resin. Nucl. Instrum. Method. Phys. Res. B 2006, 248, 31–36. [Google Scholar] [CrossRef]
- Chen, D.; Chen, F.; Hu, X.; Zhang, H.; Yin, X.; Zhou, Y. Thermal stability, mechanical and optical properties of novel addition cured PDMS composites with nano-silica sol and MQ silicone resin. Compos. Sci. Technol. 2015, 117, 307–314. [Google Scholar] [CrossRef]
- Amouroux, N.; Petit, J.; Leger, L. Role of Interfacial Resistance to Shear Stress on Adhesive Peel Strength. Langmuir 2001, 17, 6510–6517. [Google Scholar] [CrossRef]
- Xiang, H.; Ge, J.; Cheng, S.; Han, H.; Cui, S. Synthesis and characterization of titania/MQ silicone resin hybrid nanocomposite via sol-gel process. J. Sol-Gel Sci. Technol. 2011, 59, 635–639. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Z.; Yang, Y. Toughening of poly(L-lactide) with methyl MQ silicone resin. Eur. Polym. J. 2014, 50, 243–248. [Google Scholar] [CrossRef]
- Jia, P.; Liu, H.; Liu, Q.; Cai, X. Thermal degradation mechanism and flame retardancy of MQ silicon/epoxy resin composition. Polym. Degrad. Stab. 2016, 134, 144–150. [Google Scholar] [CrossRef]
- Flagg, D.H.; McCarth, Y.T.J. Rediscovering Silicones: MQ Copolymers. Macromolecules 2016, 49, 8581–8592. [Google Scholar] [CrossRef]
- Laukevic, J.J.; May, L.А.; Dreymanic, J.А.; Tutere, А.P.; Pevsner, L.J.; Vaivad, А.Y.; Katkevich, А.K. A Method of Producing of Surface-Active Silicone Polymers. U.S. Patent 176,683, 1965. [Google Scholar]
- Chugunov, V.S. Synthesis and properties of triphenylsiloxysilanes and products of their hydrolysis. Russ. Chem. Bull. 1956, 9, 1059–1061. [Google Scholar]
- Andrianov, K.A.; Severniy, V.V. The hydrolysis and condensation of trimethylsiloxychlorosilanes. Zhurnal Obsch. Chim. 1962, 32, 1633–1636. [Google Scholar]
- Ganicz, T.; Pakula, T.; Stanczyk, W.A. Novel liquid crystalline resins based on MQ siloxanes. J. Organomet. Chem. 2006, 691, 5052–5055. [Google Scholar] [CrossRef]
- Suzuki, T.; Sakae, Y.; Kushibiki, N.; Mita, I. Preparation and properties of inorgano-organiccomposite materials containing R3SiO1/2, SiO2 and TiO2 units. Chem. Mater. 1994, 6, 692–696. [Google Scholar] [CrossRef]
- Huang, W.; Huang, Y.; Yu, Y. Synthesis of MQ silicone resins through hydrolytic condensation of ethyl polysilicate and hexamethyldisiloxane. J. Appl. Polym. Sci. 1998, 70, 1753–1757. [Google Scholar] [CrossRef]
- Huang, W.; Huang, Y.; Yu, Y. The effect of the acid catalyst on the preparation of MQ silicon resins. Chin. J. Polym. Sci. 1999, 17, 429–433. [Google Scholar]
- Kuo, C.-F.J.; Chen, J.-B.; Shih, C.-Y.; Huang, C.-Y. Silicone resin synthesized by tetraethoxysilane and chlorotrimethylsilane through hydrolisis-condensation reaction. J. Appl. Polym. Sci. 2014, 131, 40317. [Google Scholar] [CrossRef]
- Altintas, Z.; Cakmack, E.; Kahraman, M.V.; Apohan, N.K. Preparation of photocurable silica–titania hybrid coatings by an anhydrous sol–gel process. Sol-Gel Sci. Technol. 2011, 58, 612–618. [Google Scholar] [CrossRef]
- Yoshinory, K.; Atsunori, M.; Masahiro, T. Formation of Anatase Nanocrystals in Sol-Gel Derived TiO2-SiO2 Thin Films with Hot Water Treatment. Sol-Gel Sci. Technol. 2000, 19, 585–588. [Google Scholar] [CrossRef]
- Berry, V.L.; Cook, L.N.; Leaym, T.M.; Schmidt, R.G. Process for the Preparation of Solid Solventless MQ Resins. U.S. Patent 8,017,712, 13 September 2011. [Google Scholar]
- Semenkova, N.J.; Nanushjan, S.R.; Storozhenko, P.A.; Polivano, A.N.; Gorjachkina, O.M. Silicon Composite Material. R.U. Patent 2,377,264, 27 December 2009. [Google Scholar]
- Chuprova, E.A.; Vinogradov, S.V.; Polivanov, A.N. Method of Producing Organosilicon Resins. R.U. Patent 2384591, 20 March 2010. [Google Scholar]
- Zeitler, V.A.; Brown, C.A. Tetrakistriphenylsiloxytitanium and Some Related Compounds. J. Am. Chem. Soc. 1957, 79, 4616–4618. [Google Scholar] [CrossRef]
- Chugunov, V.S. The syntheses of some triphenylmethyl- and trivinylcyclohexane. Russ. Chem. Bull. Ser. Chem. 1957, 11, 1368. [Google Scholar]
- Sommer, L.H.; Creen, L.Q.; Whitmore, F.C. Preparation of Organopolysiloxanes from Sodium Trimethylsilanolate. J. Am. Chem. Soc. 1949, 71, 3253–3254. [Google Scholar] [CrossRef]
- Andrianov, K.A.; Dabagova, A.K.; Syrzova, Z.S. Heterofunctional cocondensation of methyl(phenyl)acetoxysilanes with organosilicon compounds containing silicon-attached ethoxy groups. Russ. Chem. Bull. Ser. Chem. 1962, 9, 1487–1491. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Pavlov, S.P.; Dubinskaya, E.I. The interaction of trimethyliodinesilane with tetraalkoxysilane and hexaalkoxydisiloxanes. Russ. Chem. Bull. Ser. Chem. 1975, 3, 579–581. [Google Scholar] [CrossRef]
- Molchanov, B.V.; Sbrodov, A.I.; Sobolevskaya, L.V.; Chernyshev, E.A.; Chuprova, E.A.; Polivanov, A.N.; Khazanov, I.I.; Nikulina, L.S.; Chistov, S.F. A Method of Producing of Oligoorganosiloxanes. S.U. Patent 1081179, 1984. [Google Scholar]
- Lentz, C.W. Silicate minerals as sources of trimethylsilil silicates and silicate structure analysis of sodium silicate solution. Inorg. Chem. 1964, 3, 574–579. [Google Scholar] [CrossRef]
- Garzo, G.; Hoeblel, D. Gas Chromatography of trimethylsilylated silicate anions: Separation with glass capillary columns and new aspects in derivatization. J. Chromatogr. 1978, 167, 321–336. [Google Scholar] [CrossRef]
- Cervantes, J.; Rodríguez-Rodríguez, E.; Guzmán-Andrade, J.J.; Mendoza-Díaz, G.; Caudillo-González, M.; Nájera-Lara, M. Trimethylsilylation of natural silicates: Useful route toward polysiloxanes. Silicon Chem. 2003, 2, 185–194. [Google Scholar] [CrossRef]
- Caudillo-Gonzalez, M.; Sandoval, C.; Cervantes, J. Synthesis of oligosiloxane Q2M6[Q=(SiO4/2)4, M=Me3SiO2/3] from trimethylsilylation of complex silicates. Appl. Organomet. Chem. 2006, 20, 382–392. [Google Scholar] [CrossRef]
- William, H.D.; Junior, L.T. Copolymeric Siloxanes and Methods of Preparing Them. U.S. Patent 2,676,182, 13 September 1950. [Google Scholar]
- Currie, C.C.; Keil, J.W. Organopolysiloxane Adhesive and Pressure-sensitive Adhesive Tape Containing Same. U.S. Patent 2,814,601, 26 November 1957. [Google Scholar]
- Sun, F.; Hu, Y.; Du, H.-G. Synthesis and characterization of MQ silicon resin. J. Appl. Polym. Sci. 2012, 125, 3532–3536. [Google Scholar] [CrossRef]
- Andrianov, K.A.; Vasilyeva, T.V.; Dyachenko, B.I. Hydrolytic polycondensation of organochlorosilanes with silicon tetrachloride or silicic acids with a high functionality of systems. Zhurnal Obsch. Chim. 1973, XLIII, 2454–2458. [Google Scholar]
- Xu, X.; Wu, C.; Zhang, B.; Dong, H. Preparation, structure characterization, and thermal performance of phenyl-modified MQ silicone resins. J. Appl. Polym. Sci. 2013, 128, 4189–4200. [Google Scholar] [CrossRef]
- Egorova, E.V.; Vasilenko, N.G.; Demchenko, N.V.; Tatarinova, E.A.; Muzafarov, A.M. Polycondensation of Alkoxysilanes in an Active Medium as a Versatile Method for the Preparation of Polyorganosiloxanes. Dokl. Chem. 2009, 424, 15–18. [Google Scholar] [CrossRef]
- Muzafarov, A.M.; Tatarinova, E.A.; Egorova, E.V.; Meshkov, I.B. Polyphenyldimethylsiloxane Binding Agents and the Method of Their Preparation. R.U. Patent 2,422,472, 27 June 2011. [Google Scholar]
- Vasil’ev, S.G.; Volkov, V.I.; Tatarinova, E.A.; Muzafarov, A.M. A Solid-State NMR Investigation of MQ Silicone Copolymers. Appl. Magn. Reson. 2013, 44, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, K.; Yamashita, T.; Machida, S.; Horie, K.; Itoh, M.; Nishida, F.; Morino, S. Photo-probe study of siloxane polymers. I. Local free volume of an MQ-type silicon resin containing crosslinked nanoparticles probed by photoisomerization of azobenzene. J. Non-Cryst. Solids 1999, 246, 90–103. [Google Scholar] [CrossRef]
- Yoshii, K.; Machida, S.; Horie, K.; Itoh, M. Photo-probe study of siloxane polymers: Low-temperature structure relaxation in siloxane polymers probed by persistent spectral hole burning. J. Non-Cryst. Solids 2000, 272, 75–84. [Google Scholar] [CrossRef]
- Yoshii, K.; Machida, S.; Horie, K.; Itoh, M. Photo-probe study of siloxane polymers II. Local structures and dinamics of MQ-type silicone resin probed by fluorescence depolarization of perilene. Polym. J. 2000, 32, 37–42. [Google Scholar] [CrossRef]
- Chen, H.; Bujalski, D.R.; Su, K. Characterization of low molecular weight components of [ViMe2SiO1/2)x(PhSiO3/2)y(SiO4/2)z], [ViMe2SiO1/2)x (SiO4/2)z], and [(SiO4/2)x(HO1/2)y(tBuO1/2)z] silsesquioxanes by Electrospray ionization Fourier Transform Mass spectrometry (ESI-FTMS). J. Am. Soc. Mass Spectrom. 2005, 16, 524–534. [Google Scholar] [CrossRef]
- Li, H.-L.; Ujihira, Y.; Yoshii, K.; Yamashita, T.; Horie, K. Free volumes and their distribution in crosslinked polysiloxanes probed by positron annihilation lifetime technique. Polymer 1998, 39, 4075–4079. [Google Scholar] [CrossRef]
- Mironova, M.V.; Tatarinova, E.A.; Meshkov, I.B.; Muzafarov, A.M.; Kulichikhin, V.G. Rheological and relaxation properties of MQ copolymers. Polym. Sci. Ser. A 2012, 54, 177–186. [Google Scholar] [CrossRef]
- Muzafarov, A.M.; Vasilenko, N.G.; Shragin, D.I. Chlorine-free Chemistry of Silicones—A New Reality; “Pero”: Moscow, Russia, 2016; ISBN 978-5-906909-92-3. [Google Scholar]
- Jayes, L.; Hard, A.P.; Sene, C.; Parker, S.F.; Jayasooriya, U.A. Vibrational Spectroscopic Analysis of Silicones: A Fourier Transform-Raman and Inelastic Neutron Scattering Investigation. Anal. Chem. 2003, 75, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Vasil’ev, S.G.; Volkov, V.I.; Tatarinova, E.A.; Muzafarov, A.M. Study of Self-Diffusion of Silicone MQ Resins in Chloroform Solutions by Pulsed Field-Gradient NMR Spectroscopy. Appl. Magn. Reson. 2014, 45, 315–328. [Google Scholar] [CrossRef]
- Voronina, N.V.; Meshkov, I.B.; Myakushev, V.D.; Laptinskaya, T.V.; Papkov, V.S.; Buzin, M.I.; Il’ina, M.N.; Ozerin, A.N.; Muzafarov, A.M. Hybrid Organo-Inorganic Globular Nanospecies: Transition from Macromolecule to Particle. J. Polym. Sci. Part A 2010, 48, 4310–4322. [Google Scholar] [CrossRef]
- Arkles, B. Commercial Applications of Sol-Gel-Derived Hybrid Materials. MRS Bull. 2001, 26, 402–407. [Google Scholar] [CrossRef]
Sample Availability: Samples of the MQ copolymers are available from the authors. |








| Sample, No. | M × 10−3 (GPC) | Content of OH-Groups, % Mass | Rhydr, nm (GPC) | Tg, °C | η, Pa·s |
|---|---|---|---|---|---|
| MQ1 | 3.5 | 2.26 | 1.30 | 55 | 1.2 × 106 |
| MQ2 | 1.5 | 0.97 | 0.80 | 10 | 1.0 × 102 |
| MQ3 | 3.5 | 2.7 | 1.30 | 61 | 1.5 × 105 |
| MQ4 | 1.7 | 1.4 | 0.86 | 34 | 5.6 × 103 |
| MQ5 | 3.5 | 2.8 | 1.30 | 46 | 4.7 × 104 |
| MQ6 | 3.5 | 0 | 1.30 | 71 | 1.4 × 106 |
| Fraction Yield% | MM, GPC | Tg °C | ||
|---|---|---|---|---|
| MQ1 | Fr. 1 | 28 | 7600 | >Tdecomp.* |
| Fr. 2 | 44 | 3900 | 160 | |
| Fr. 3 | 28 | 2000 | −26 | |
| MQ2 | Fr. 1 | 11 | 5400 | 319 |
| Fr. 2 | 39 | 2700 | 78 | |
| Fr. 3 | 50 | 1500 | 38 | |
| MQ3 | Fr. 1 | 20 | 5800 | >Tdecomp. |
| Fr. 2 | 45 | 3000 | 110 | |
| Fr. 3 | 35 | 1500 | −10 | |
| MQ4 | Fr. 1 | 19 | 5300 | 273 |
| Fr. 2 | 39 | 3000 | 110 | |
| Fr. 3 | 42 | 1500 | −28 | |
| MQ5 | Fr. 1 | 21 | 11,000 | >Tdecomp. |
| Fr. 2 | 45 | 3900 | 180 | |
| Fr. 3 | 34 | 1200 | −24 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatarinova, E.; Vasilenko, N.; Muzafarov, A. Synthesis and Properties of MQ Copolymers: Current State of Knowledge. Molecules 2017, 22, 1768. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22101768
Tatarinova E, Vasilenko N, Muzafarov A. Synthesis and Properties of MQ Copolymers: Current State of Knowledge. Molecules. 2017; 22(10):1768. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22101768
Chicago/Turabian StyleTatarinova, Elena, Nataliya Vasilenko, and Aziz Muzafarov. 2017. "Synthesis and Properties of MQ Copolymers: Current State of Knowledge" Molecules 22, no. 10: 1768. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22101768