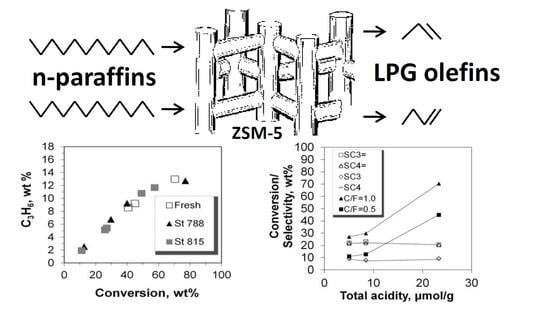
Effect of Steam Deactivation Severity of ZSM-5 Additives on LPG Olefins Production in the FCC Process
Abstract
:1. Introduction
2. Results and Discussion
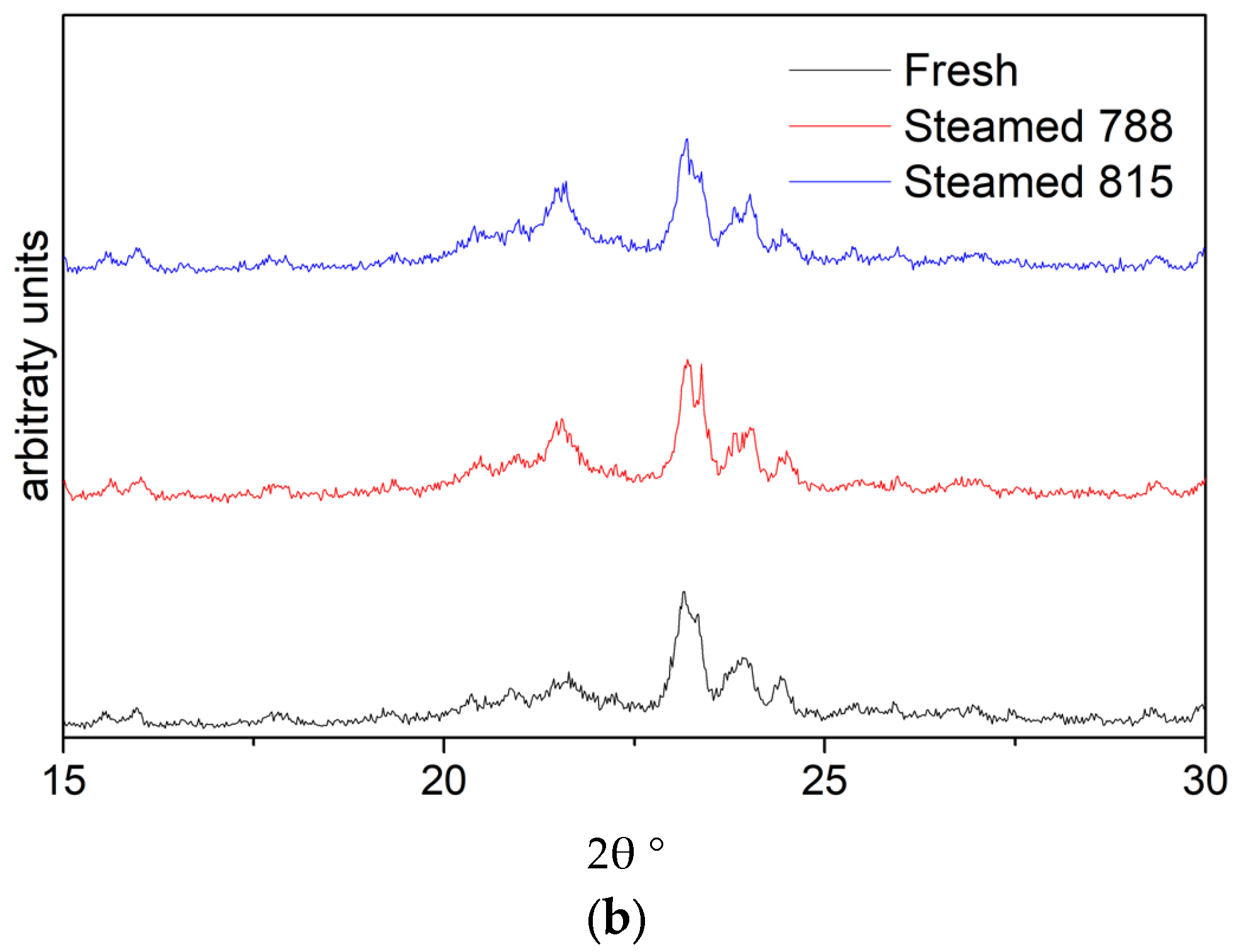
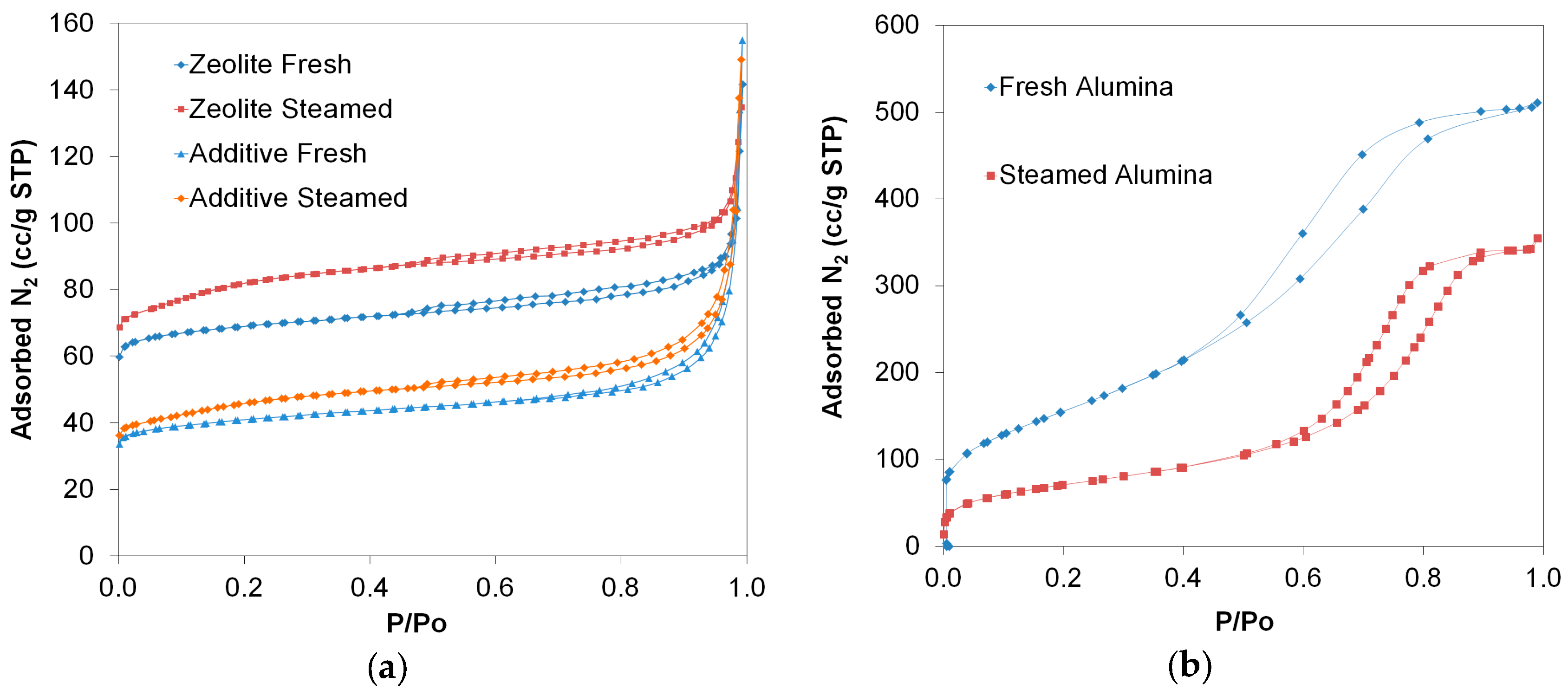
2.1. Structural, Porosity, and Acidic Characteristics of ZSM-5 Additives
2.2. Cracking of n-Dodecane (n-C12)
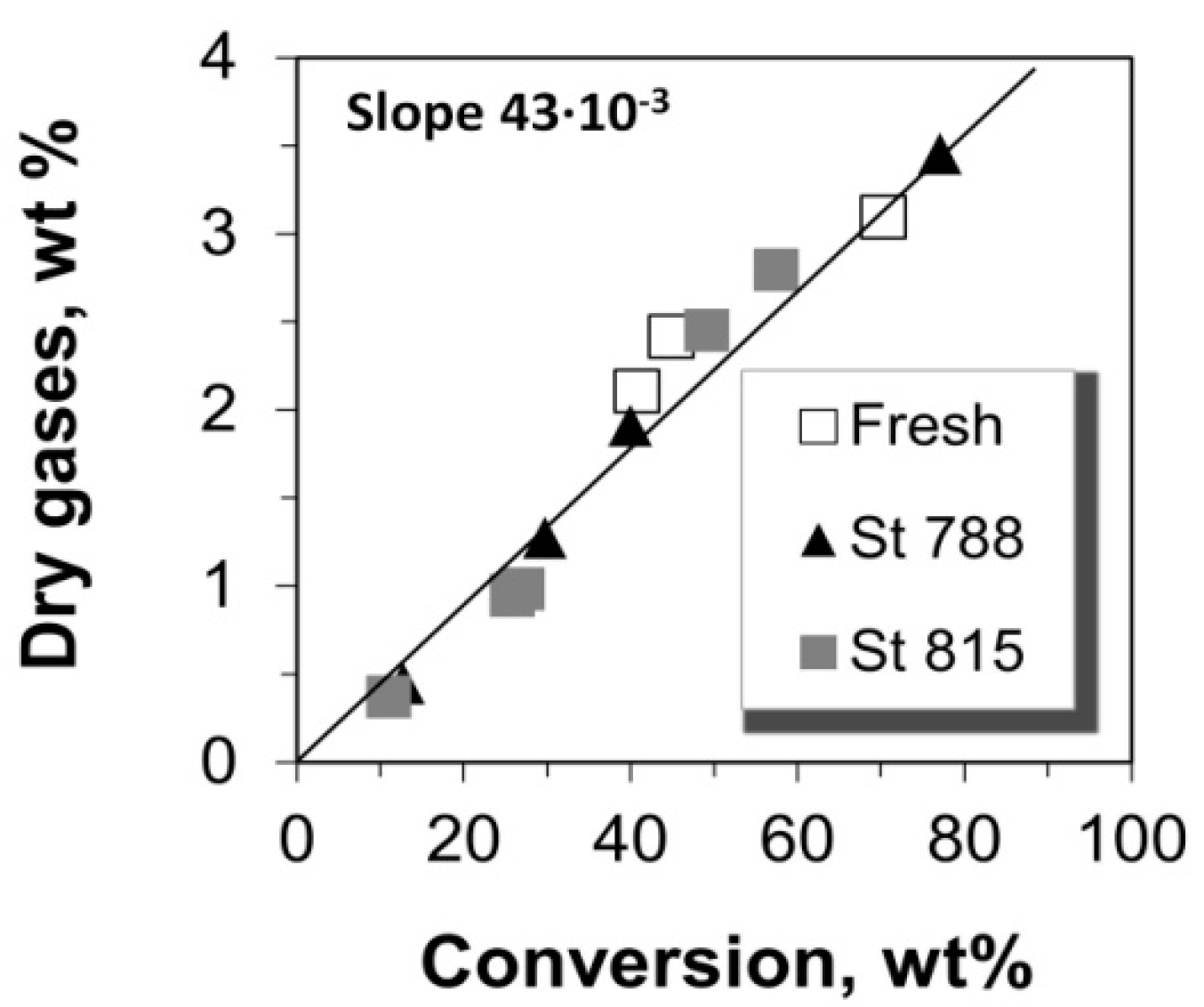
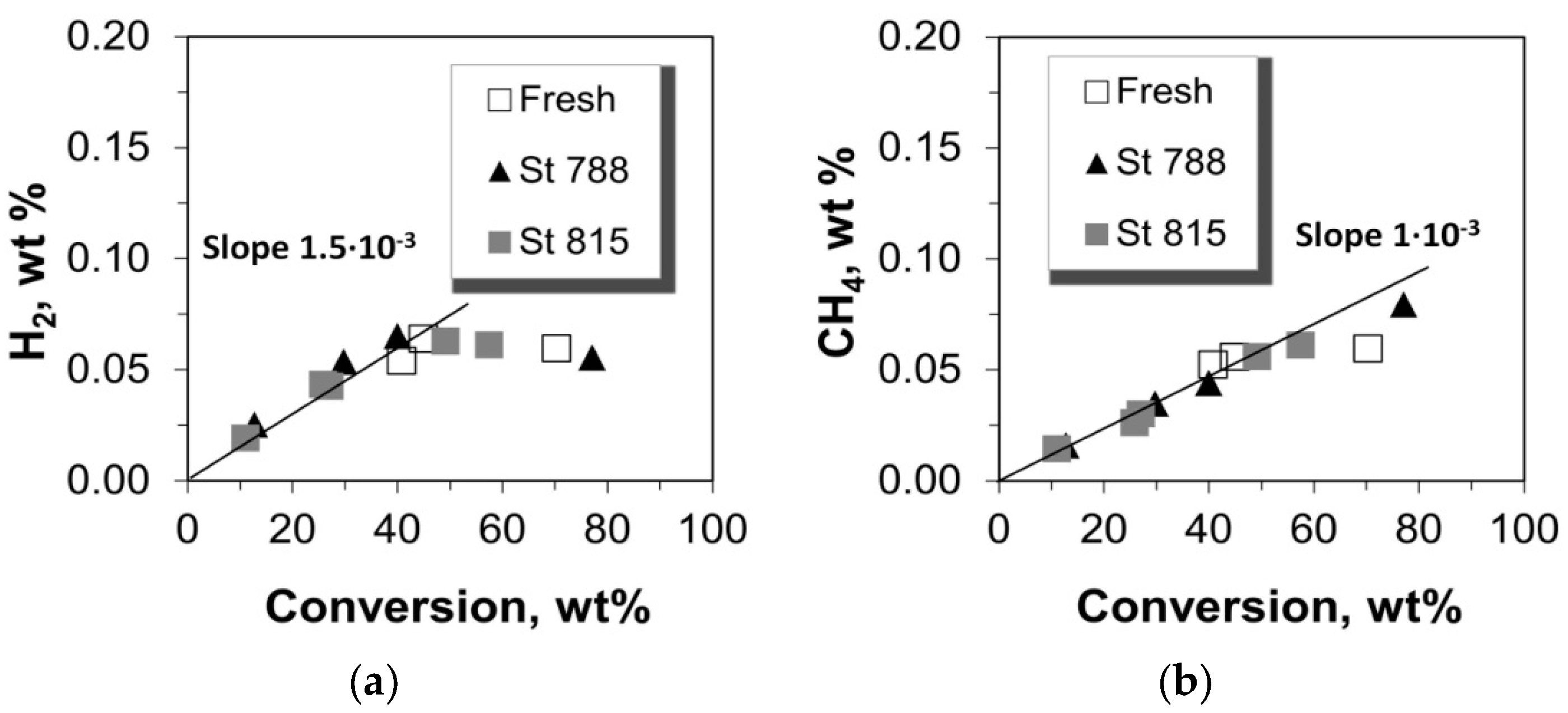
2.3. Selectivity to Dry Gases
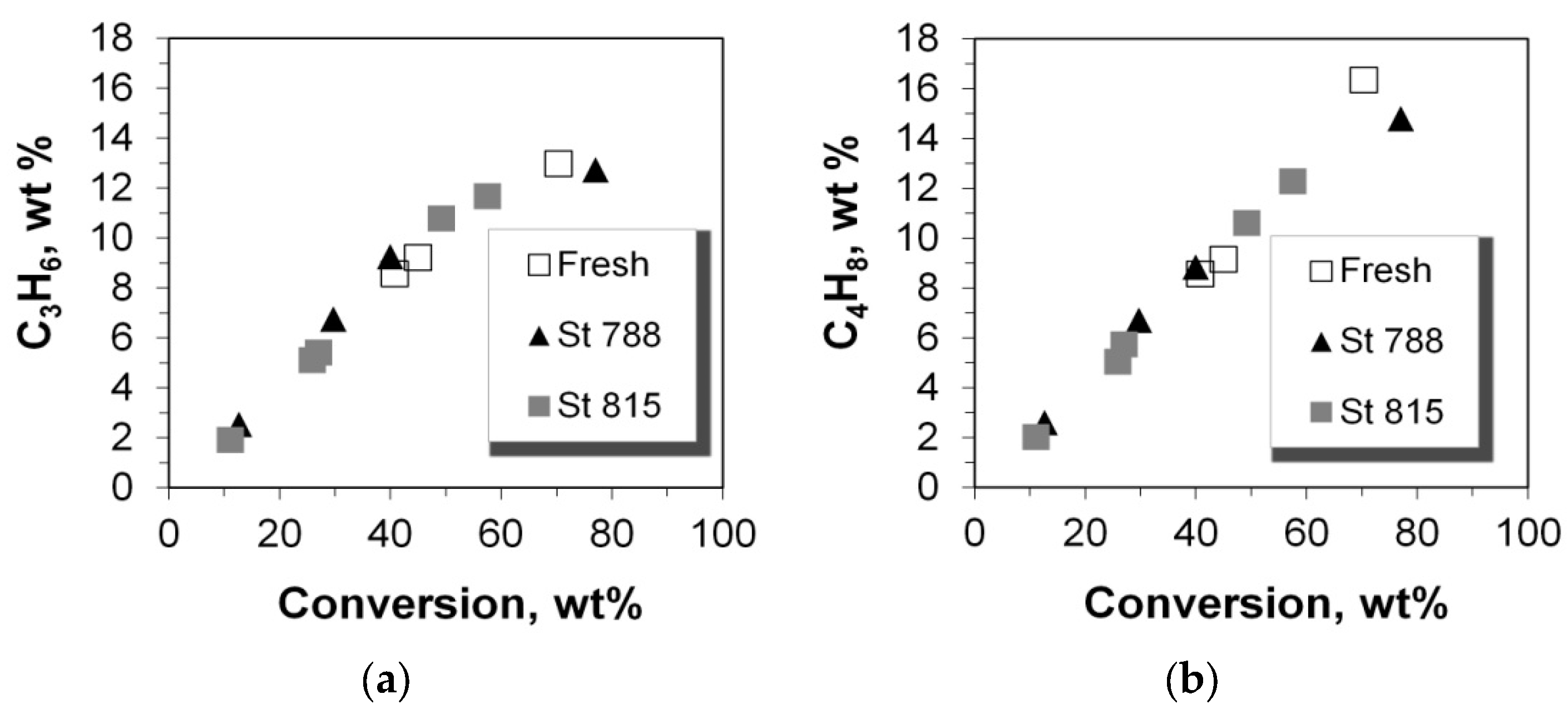
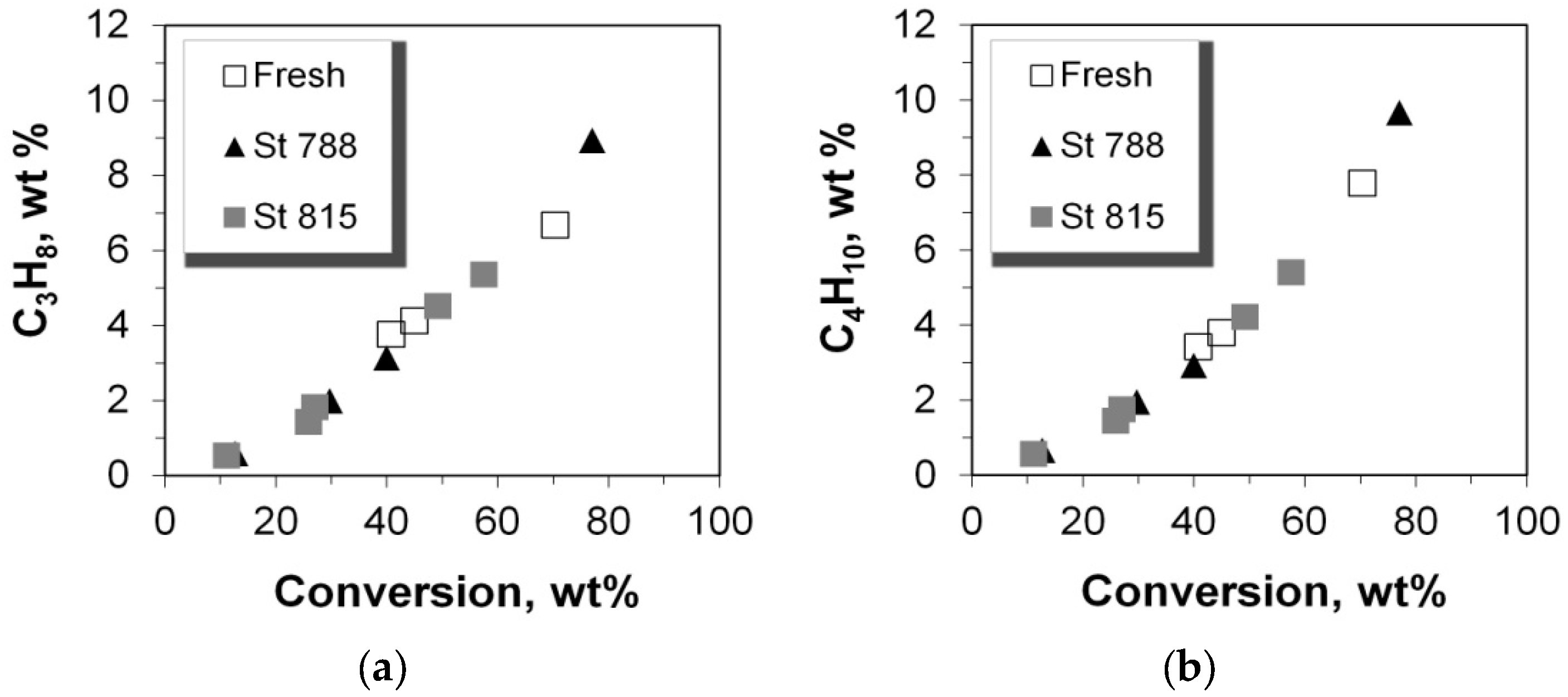
2.4. Selectivity of LPG Products
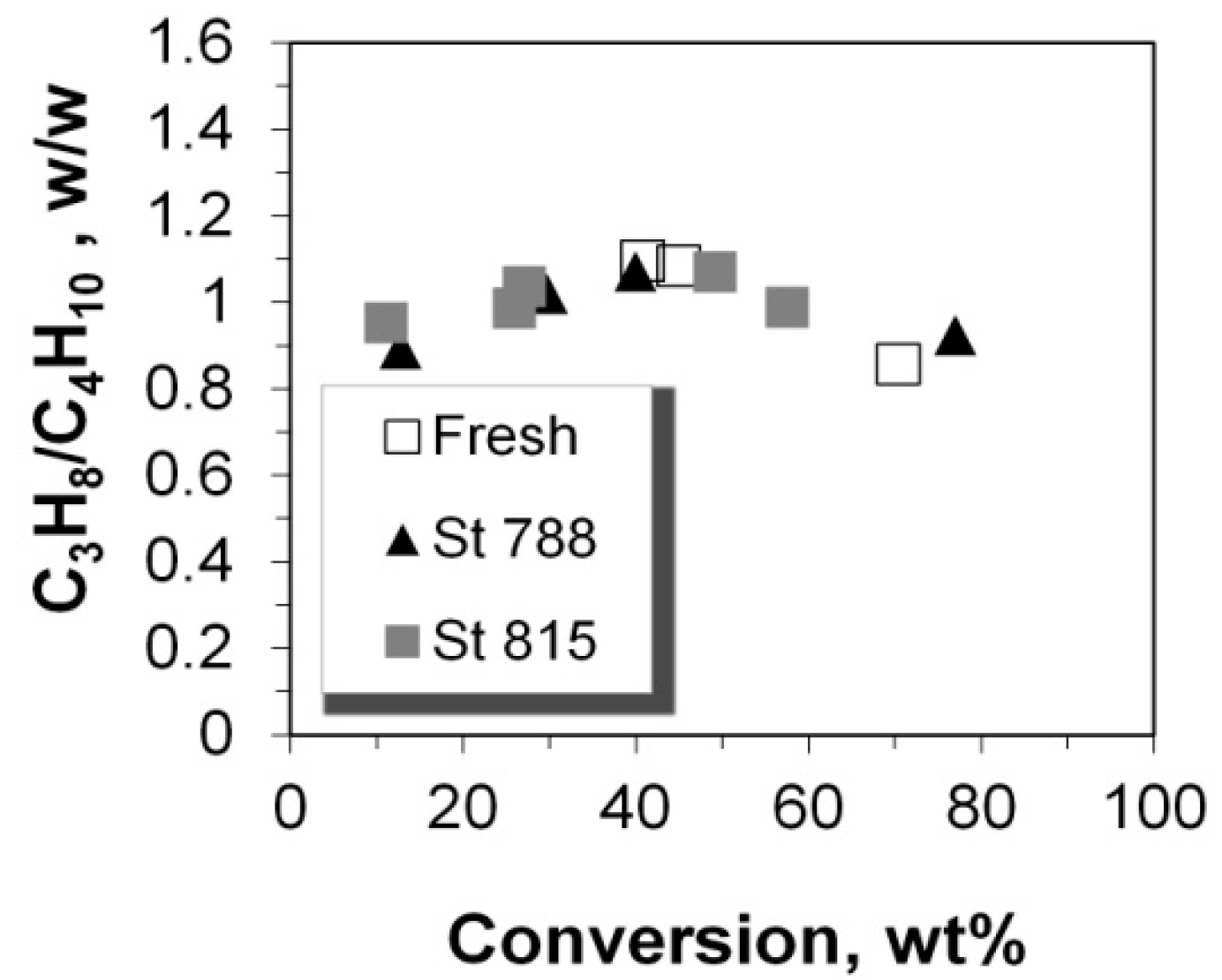
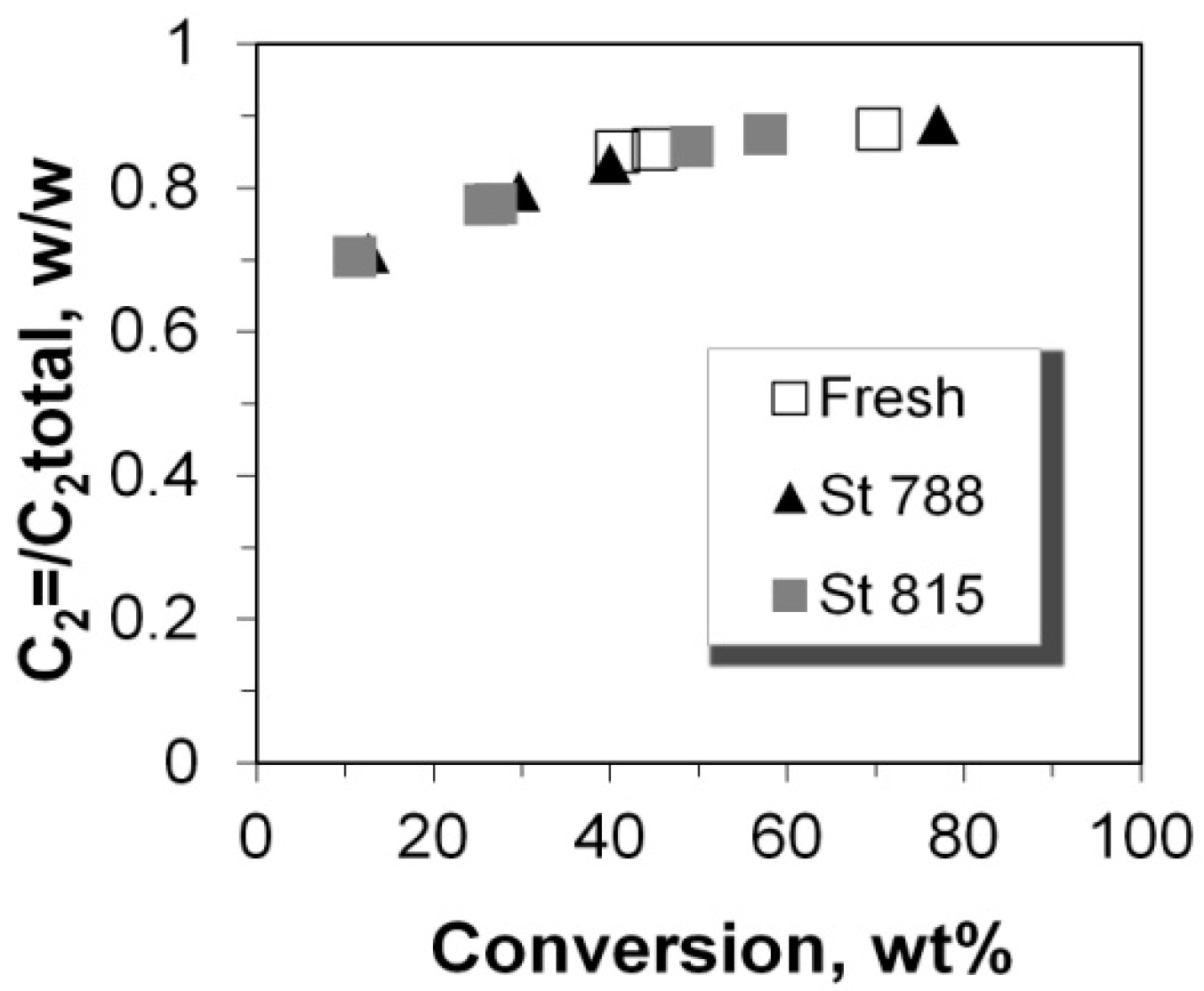
2.5. C2–C5 Olefinicities
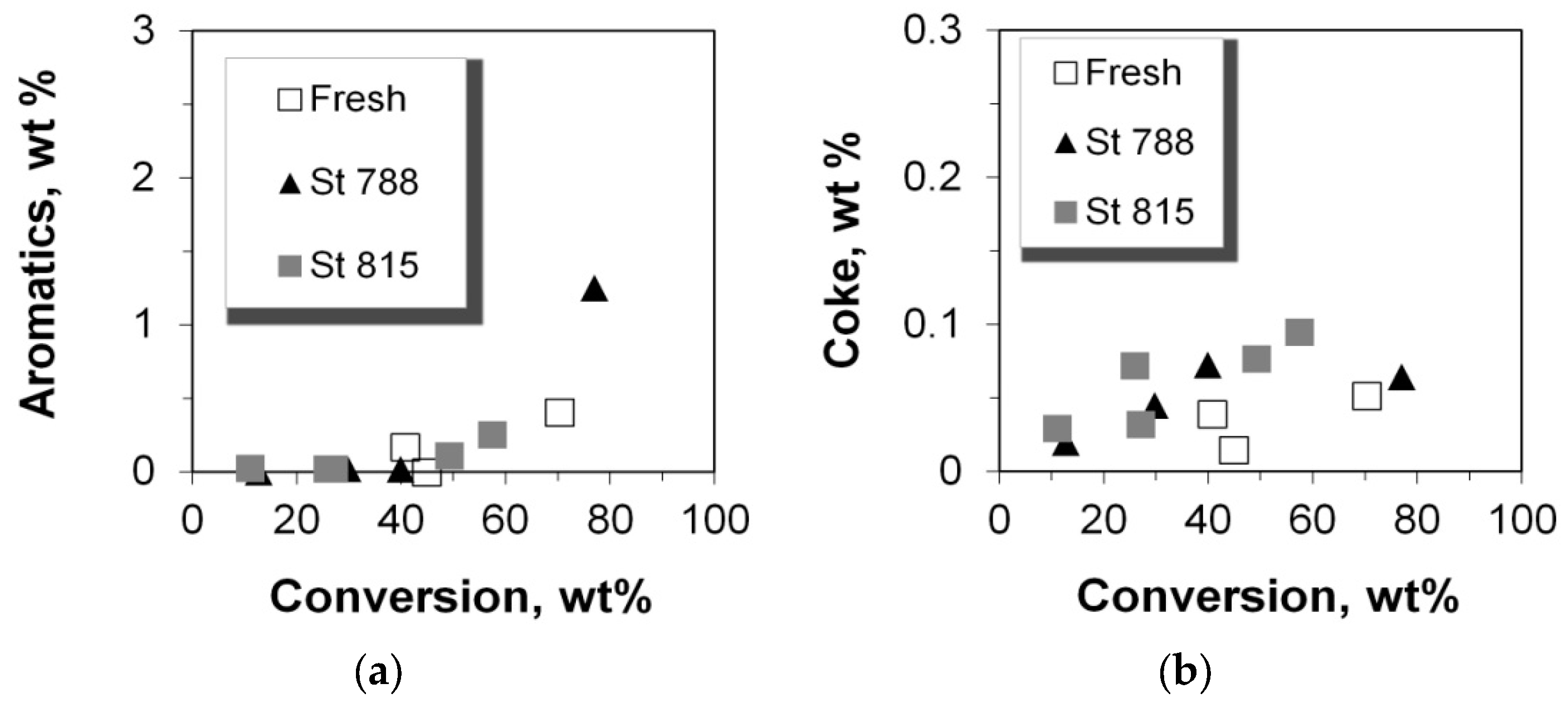
2.6. Production of Coke and Aromatics
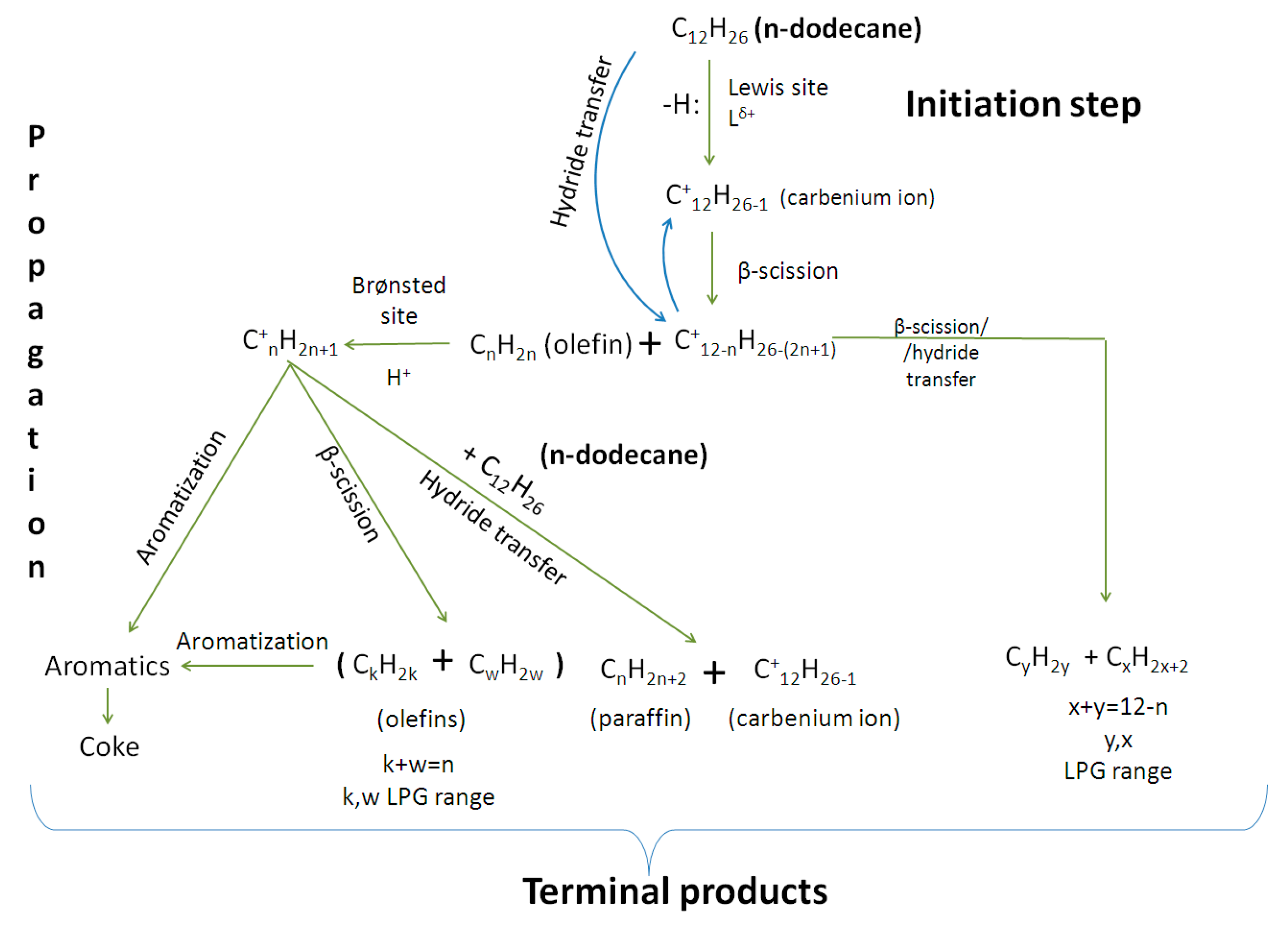
2.7. Cracking Mechanism
3. Experimental
3.1. Catalysts
3.2. Characterization
3.3. Catalytic Cracking Experiments
3.4. Product Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malgras, V.; Ji, Q.; Kamachi, Y.; Mori, T.; Shieh, F.-K.; Wu, K.C.-W.; Ariga, K.; Yamauchi, Y. Templated Synthesis for Nanoarchitectured Porous Materials. Bull. Chem. Soc. Jpn. 2015, 88, 1171–1200. [Google Scholar] [CrossRef]
- Shieh, F.-K.; Wang, S.-C.; Yen, C.-I.; Wu, C.-C.; Dutta, S.; Chou, L.-Y.; Morabito, J.V.; Hu, P.; Hsu, M.-H.; Wu, K.C.-W.; et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal–Organic Framework Microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.H.; Haag, W.O.; Lago, R.M. Chemical and physical properties of the ZSM-5 substitutional series. J. Catal. 1980, 61, 390–396. [Google Scholar] [CrossRef]
- Haag, W.O.; Lago, R.M.; Weisz, P.B. Transport and reactivity of hydrocarbon molecules in a shape-selective zeolite. Faraday Discuss. Chem. Soc. 1981, 72, 317–330. [Google Scholar] [CrossRef]
- Olson, D.H.; Kokotailo, G.T.; Lawton, S.L.; Meier, W.M. Crystal structure and structure-related properties of ZSM-5. J. Phys. Chem. 1981, 85, 2238–2243. [Google Scholar] [CrossRef]
- Tabak, S.A.; Krambeck, F.J.; Garwood, W.E. Conversion of propylene and butylene over ZSM-5 catalyst. AIChE J. 1986, 32, 1526–1531. [Google Scholar] [CrossRef]
- Biswas, J.; Maxwell, I.E. Octane enhancement in fluid catalytic cracking: II. Operation in the overcracking regime. Appl. Catal. 1990, 58, 19–27. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N.S.; Alario, F. Aromatization of short chain alkanes on zeolite catalysts. Appl. Catal. A Gen. 1992, 89, 1–30. [Google Scholar] [CrossRef]
- Triantafillidis, C.S.; Evmiridis, N.P.; Nalbandian, L.; Vasalos, I.A. Performance of ZSM-5 as a Fluid Catalytic Cracking Catalyst Additive: Effect of the Total Number of Acid Sites and Particle Size. Ind. Eng. Chem. Res. 1999, 38, 916–927. [Google Scholar] [CrossRef]
- Buchanan, J.S. The chemistry of olefins production by ZSM-5 addition to catalytic cracking units. Catal. Today 2000, 55, 207–212. [Google Scholar] [CrossRef]
- Wallenstein, D.; Harding, R.H. The dependence of ZSM-5 additive performance on the hydrogen-transfer activity of the REUSY base catalyst in fluid catalytic cracking. Appl. Catal. A Gen. 2001, 214, 11–29. [Google Scholar] [CrossRef]
- Buchanan, J.S. Reactions of model compounds over steamed ZSM-5 at simulated FCC reaction conditions. Appl. Catal. 1991, 74, 83–94. [Google Scholar] [CrossRef]
- Pimenta, R.D.M.; Pereira, M.M.; do Nascimento, U.; Gorne, J.; Bernadete, E.; Lau, L.Y. Effect of vanadium contamination on H-ZSM-5 zeolite deactivation. Catal. Today 2008, 133–135, 805–808. [Google Scholar] [CrossRef]
- Corma, A.; Miguel, P.J.; Orchillés, A.V. Product selectivity effects during cracking of alkanes at very short and longer times on stream. Appl. Catal. A Gen. 1996, 138, 57–73. [Google Scholar] [CrossRef]
- Van Bokhoven, J.A.; Williams, B.A.; Ji, W.; Koningsberger, D.C.; Kung, H.H.; Miller, J.T. Observation of a compensation relation for monomolecular alkane cracking by zeolites: The dominant role of reactant sorption. J. Catal. 2004, 224, 50–59. [Google Scholar] [CrossRef]
- Altwasser, S.; Welker, C.; Traa, Y.; Weitkamp, J. Catalytic cracking of n-octane on small-pore zeolites. Microporous Mesoporous Mater. 2005, 83, 345–356. [Google Scholar] [CrossRef]
- Gounder, R.; Iglesia, E. Catalytic Consequences of Spatial Constraints and Acid Site Location for Monomolecular Alkane Activation on Zeolites. J. Am. Chem. Soc. 2009, 131, 1958–1971. [Google Scholar] [CrossRef] [PubMed]
- Rownaghi, A.A.; Rezaei, F.; Hedlund, J. Selective formation of light olefin by n-hexane cracking over HZSM-5: Influence of crystal size and acid sites of nano- and micrometer-sized crystals. Chem. Eng. J. 2012, 191, 528–533. [Google Scholar] [CrossRef]
- Lappas, A.A.; Iatridis, D.K.; Vasalos, I.A. Production of reformulated gasoline in FCC unit. Effect of feedstock type on gasoline composition. Catal. Today 1999, 50, 73–85. [Google Scholar] [CrossRef]
- Buchanan, J.S.; Adewuyi, Y.G. Effects of high temperature and high ZSM-5 addtive level on FCC olefins yields and gasoline composition. Appl. Catal. A Gen. 1996, 134, 247–262. [Google Scholar] [CrossRef]
- Zhao, X.; Roberie, T.G. ZSM-5 Additive in Fluid Catalytic Cracking. 1. Effect of Additive Level and Temperature on Light Olefins and Gasoline Olefins. Ind. Eng. Chem. Res. 1999, 38, 3847–3853. [Google Scholar] [CrossRef]
- Zhao, X.; Harding, R.H. ZSM-5 Additive in Fluid Catalytic Cracking. 2. Effect of Hydrogen Transfer Characteristics of the Base Cracking Catalysts and Feedstocks. Ind. Eng. Chem. Res. 1999, 38, 3854–3859. [Google Scholar] [CrossRef]
- Lappas, A.A.; Triantafillidis, C.S.; Tsagrasouli, Z.A.; Tsiatouras, V.A.; Vasalos, I.A.; Evmiridis, N.P. Development of new ZSM-5 catalyst-additives in the Fluid Catalytic Cracking for the maximization of gaseous alkenes yield. Stud. Surf. Sci. Catal. 2002, 142, 807–814. [Google Scholar]
- Adewuyi, Y.G. Compositional changes in FCC gasoline products resulting from high-level additions of ZSM-5 zeolite to RE-USY catalyst. Appl. Catal. A Gen. 1997, 163, 15–29. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Klocke, D.J.; Buchanan, J.S. Effects of high-level additions of ZSM-5 to a fluid catalytic cracking (FCC) RE-USY catalyst. Appl. Catal. A Gen. 1995, 131, 121–133. [Google Scholar] [CrossRef]
- Aitani, A.; Yoshikawa, T.; Ino, T. Maximization of FCC light olefins by high severity operation and ZSM-5 addition. Catal. Today 2000, 60, 111–117. [Google Scholar] [CrossRef]
- Buchanan, J.S. Gasoline selective ZSM-5 FCC additives: Model reactions of C 6–C 10 olefins over steamed 55: 1 and 450: 1 ZSM-5. Appl. Catal. A Gen. 1998, 171, 57–64. [Google Scholar] [CrossRef]
- Buchanan, J.S.; Olson, D.H.; Schramm, S.E. Gasoline selective ZSM-5 FCC additives: Effects of crystal size, SiO2/Al2O3, steaming, and other treatments on ZSM-5 diffusivity and selectivity in cracking of hexene/octene feed. Appl. Catal. A Gen. 2001, 220, 223–234. [Google Scholar] [CrossRef]
- Biswas, J.; Maxwell, I.E. Octane enhancement in fluid catalytic cracking: I. Role of ZSM-5 addition and reactor temperature. Appl. Catal. 1990, 58, 1–18. [Google Scholar] [CrossRef]
- Knight, J.; Mehlberg, R. Maximize propylene from FCC unit. Hydrocarb. Process. September 2011; 91–95. [Google Scholar]
- Nalbandian, L.; Lemonidou, A.A.; Vasalos, I.A. Microactivity test (MAT) study of the ZSM-5 addition effects on FCC product yields and gasoline composition. Appl. Catal. A Gen. 1993, 105, 107–125. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Coking and deactivation of zeolites: Influence of the pore structure. Appl. Catal. 1989, 54, 1–27. [Google Scholar] [CrossRef]
- Cerqueira, H.S.; Caeiro, G.; Costa, L.; Ramôa Ribeiro, F. Deactivation of FCC catalysts. J. Mol. Catal. Chem. 2008, 292, 1–13. [Google Scholar] [CrossRef]
- Sano, T.; Ikeya, H.; Kasuno, T.; Wang, Z.B.; Kawakami, Y.; Soga, K. Influence of crystallinity of HZSM-5 zeolite on its dealumination rate. Zeolites 1997, 19, 80–86. [Google Scholar] [CrossRef]
- Silaghi, M.-C.; Chizallet, C.; Sauer, J.; Raybaud, P. Dealumination mechanisms of zeolites and extra-framework aluminum confinement. J. Catal. 2016, 339, 242–255. [Google Scholar] [CrossRef]
- Guerzoni, F.N.; Abbot, J. Cracking of an industrial feedstock over combinations of H-ZSM-5 and HY: The influence of H-ZSM-5 pretreatment. Appl. Catal. A Gen. 1994, 120, 55–69. [Google Scholar] [CrossRef]
- Auroux, A. Acidity characterization by microcalorimetry and relationship with reactivity. Top. Catal. 1997, 4, 71–89. [Google Scholar] [CrossRef]
- Rautiainen, E.; Pimenta, R.; Ludvig, M.; Pouwels, C. Deactivation of ZSM-5 additives in laboratory for realistic testing. Catal. Today 2009, 140, 179–186. [Google Scholar] [CrossRef]
- Ong, L.H.; Dömök, M.; Olindo, R.; van Veen, A.C.; Lercher, J.A. Dealumination of HZSM-5 via steam-treatment. Microporous Mesoporous Mater. 2012, 164, 9–20. [Google Scholar] [CrossRef]
- Liu, D.; Choi, W.C.; Lee, C.W.; Kang, N.Y.; Lee, Y.J.; Shin, C.-H.; Park, Y.K. Steaming and washing effect of P/HZSM-5 in catalytic cracking of naphtha. Catal. Today 2011, 164, 154–157. [Google Scholar] [CrossRef]
- Aramburo, L.R.; Karwacki, L.; Cubillas, P.; Asahina, S.; de Winter, D.A.M.; Drury, M.R.; Buurmans, I.L.C.; Stavitski, E.; Mores, D.; Daturi, M.; et al. The Porosity, Acidity, and Reactivity of Dealuminated Zeolite ZSM-5 at the Single Particle Level: The Influence of the Zeolite Architecture. Chem. Eur. J. 2011, 17, 13773–13781. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Mengual, J.; Miguel, P.J. Stabilization of ZSM-5 zeolite catalysts for steam catalytic cracking of naphtha for production of propene and ethene. Appl. Catal. A Gen. 2012, 421–422, 121–134. [Google Scholar] [CrossRef]
- Mante, O.D.; Agblevor, F.A.; Oyama, S.T.; McClung, R. The effect of hydrothermal treatment of FCC catalysts and ZSM-5 additives in catalytic conversion of biomass. Appl. Catal. A Gen. 2012, 445–446, 312–320. [Google Scholar] [CrossRef]
- Aramburo, L.R.; Ruiz-Martínez, J.; Hofmann, J.P.; Weckhuysen, B.M. Imaging the effect of a hydrothermal treatment on the pore accessibility and acidity of large ZSM-5 zeolite crystals by selective staining. Catal. Sci. Technol. 2013, 3, 1208–1214. [Google Scholar] [CrossRef]
- Corma, A.; Mengual, J.; Miguel, P.J. Steam catalytic cracking of naphtha over ZSM-5 zeolite for production of propene and ethene: Micro and macroscopic implications of the presence of steam. Appl. Catal. A Gen. 2012, 417–418, 220–235. [Google Scholar] [CrossRef]
- Corma, A.; Mengual, J.; Miguel, P.J. IM-5 zeolite for steam catalytic cracking of naphtha to produce propene and ethene. An alternative to ZSM-5 zeolite. Appl. Catal. A Gen. 2013, 460–461, 106–115. [Google Scholar] [CrossRef]
- Kubo, K.; Iida, H.; Namba, S.; Igarashi, A. Ultra-high steaming stability of Cu-ZSM-5 zeolite as naphtha cracking catalyst to produce light olefin. Catal. Commun. 2012, 29, 162–165. [Google Scholar] [CrossRef]
- Kubo, K.; Iida, H.; Namba, S.; Igarashi, A. Effect of steaming on acidity and catalytic performance of H-ZSM-5 and P/H-ZSM-5 as naphtha to olefin catalysts. Microporous Mesoporous Mater. 2014, 188, 23–29. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Jin, D.; Ikeda, T.; Sato, K.; Hiyoshi, N.; Hanaoka, T.; Mizukami, F.; Shirai, M. Deactivation of ZSM-5 zeolite during catalytic steam cracking of n-hexane. Fuel Process. Technol. 2014, 126, 343–349. [Google Scholar] [CrossRef]
- Niwa, M.; Sota, S.; Katada, N. Strong Brønsted acid site in HZSM-5 created by mild steaming. Catal. Today 2012, 185, 17–24. [Google Scholar] [CrossRef]
- Kubo, K.; Iida, H.; Namba, S.; Igarashi, A. Comparison of steaming stability of Cu-ZSM-5 with those of Ag-ZSM-5, P/H-ZSM-5, and H-ZSM-5 zeolites as naphtha cracking catalysts to produce light olefin at high temperatures. Appl. Catal. A Gen. 2015, 489, 272–279. [Google Scholar] [CrossRef]
- Blasco, T.; Corma, A.; Martineztriguero, J. Hydrothermal stabilization of ZSM-5 catalytic-cracking additives by phosphorus addition. J. Catal. 2006, 237, 267–277. [Google Scholar] [CrossRef]
- Sendoda, Y.; Ono, Y. Effect of the pretreatment temperature on the catalytic activity of ZSM-5 zeolites. Zeolites 1988, 8, 101–105. [Google Scholar] [CrossRef]
- Jentoft, F.C.; Gates, B.C. Solid-acid-catalyzed alkane cracking mechanisms: Evidence from reactions of small probe molecules. Top. Catal. 1197, 4, 1–13. [Google Scholar] [CrossRef]
- Lukyanov, D.B.; Shtral, V.I.; Khadzhiev, S.N. A kinetic model for the hexane cracking reaction over H-ZSM-5. J. Catal. 1994, 146, 87–92. [Google Scholar] [CrossRef]
- Krannila, H.; Haag, W.O.; Gates, B.C. Monomolecular and bimolecular mechanisms of paraffin cracking: N-butane cracking catalyzed by HZSM-5. J. Catal. 1992, 135, 115–124. [Google Scholar] [CrossRef]
- Narbeshuber, T.F.; Vinek, H.; Lercher, J.A. Monomolecular conversion of light alkanes over H-ZSM-5. J. Catal. 1995, 157, 388–395. [Google Scholar] [CrossRef]
- Babitz, S.M.; Williams, B.A.; Miller, J.T.; Snurr, R.Q.; Haag, W.O.; Kung, H.H. Monomolecular cracking of n-hexane on Y, MOR, and ZSM-5 zeolites. Appl. Catal. A Gen. 1999, 179, 71–86. [Google Scholar] [CrossRef]
- Jung, J.S.; Park, J.W.; Seo, G. Catalytic cracking of n-octane over alkali-treated MFI zeolites. Appl. Catal. A Gen. 2005, 288, 149–157. [Google Scholar] [CrossRef]
- Konno, H.; Tago, T.; Nakasaka, Y.; Ohnaka, R.; Nishimura, J.; Masuda, T. Effectiveness of nano-scale ZSM-5 zeolite and its deactivation mechanism on catalytic cracking of representative hydrocarbons of naphtha. Microporous Mesoporous Mater. 2013, 175, 25–33. [Google Scholar] [CrossRef]
- Urata, K.; Furukawa, S.; Komatsu, T. Location of coke on H-ZSM-5 zeolite formed in the cracking of n-hexane. Appl. Catal. A Gen. 2014, 475, 335–340. [Google Scholar] [CrossRef]
- Kubo, K.; Takahashi, T.; Iida, H.; Namba, S.; Igarashi, A. Reactivities of C6–8 hydrocarbons and effect of coexistence of another hydrocarbon in cracking on H-ZSM-5 catalyst at high temperatures. Appl. Catal. A Gen. 2014, 482, 370–376. [Google Scholar] [CrossRef]
- Janda, A.; Bell, A.T. Effects of Si/Al Ratio on the Distribution of Framework Al and on the Rates of Alkane Monomolecular Cracking and Dehydrogenation in H-MFI. J. Am. Chem. Soc. 2013, 135, 19193–19207. [Google Scholar] [CrossRef] [PubMed]
- Schallmoser, S.; Ikuno, T.; Wagenhofer, M.F.; Kolvenbach, R.; Haller, G.L.; Sanchez-Sanchez, M.; Lercher, J.A. Impact of the local environment of Brønsted acid sites in ZSM-5 on the catalytic activity in n-pentane cracking. J. Catal. 2014, 316, 93–102. [Google Scholar] [CrossRef]
- Li, H.; Kadam, S.A.; Vimont, A.; Wormsbecher, R.F.; Travert, A. Monomolecular Cracking Rates of Light Alkanes over Zeolites Determined by IR Operando Spectroscopy. ACS Catal. 2016, 6, 4536–4548. [Google Scholar] [CrossRef]
- Wielers, A.F.H.; Vaarkamp, M.; Post, M.F.M. Relation between properties and performance of zeolites in paraffin cracking. J. Catal. 1991, 127, 51–66. [Google Scholar] [CrossRef]
- Sanhoob, M.A.; Muraza, O.; Shafei, E.N.; Yokoi, T.; Choi, K.-H. Steam catalytic cracking of heavy naphtha (C12) to high octane naphtha over B-MFI zeolite. Appl. Catal. B Environ. 2017, 210, 432–443. [Google Scholar] [CrossRef]
- Liu, D.; Choi, W.C.; Kang, N.Y.; Lee, Y.J.; Park, H.S.; Shin, C.-H.; Park, Y.-K. Inter-conversion of light olefins on ZSM-5 in catalytic naphtha cracking condition. Catal. Today 2014, 226, 52–66. [Google Scholar] [CrossRef]
- Haag, W.O.; Dessau, R.M. Duality of mechanism for acid-catalyzed paraffin cracking. In Proceedings of 8th International Congress on Catalysis; Dechema, Frankfurt am Main: Berlin, Germany, 1984; Volume 2, p. 305. [Google Scholar]
- Corma, A.; Planelles, J.; Sánchez-Marín, J.; Tomas, F. The role of different types of acid site in the cracking of alkanes on zeolite catalysts. J. Catal. 1985, 93, 30–37. [Google Scholar] [CrossRef]
- Sie, S.T. Acid-catalyzed cracking of paraffinic hydrocarbons. 1. Discussion of existing mechanisms and proposal of a new mechanism. Ind. Eng. Chem. Res. 1992, 31, 1881–1889. [Google Scholar] [CrossRef]
- Kotrel, S.; Knözinger, H.; Gates, B.C. The Haag–Dessau mechanism of protolytic cracking of alkanes. Microporous Mesoporous Mater. 2000, 35, 11–20. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Frash, M.V.; Van Santen, R.A. A quantum-chemical study of adsorbed nonclassical carbonium ions as active intermediates in catalytic transformations of paraffins. II. Protolytic dehydrogenation and hydrogen-deuterium hetero-isotope exchange of paraffins on high-silica zeolites. Catal. Lett. 1994, 28, 211–222. [Google Scholar] [CrossRef]
- Blaszkowsky, S.R.; Van Santen, R.A. Quantum chemical studies of zeolite proton catalyzed reactions. Top. Catal. 1997, 4, 145–156. [Google Scholar] [CrossRef]
- Triantafillidis, C.S.; Vlessidis, A.G.; Nalbandian, L.; Evmiridis, N.P. Effect of degree and type of the dealumination method on the structural, compositional and acidic characteristics of H-ZSM-5 zeolites. Microporous Mesoporous Mater. 2001, 47, 369–388. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87. [Google Scholar] [CrossRef]
- Topsøe, N.-Y.; Joensen, F.; Derouane, E.G. IR studies of the nature of the acid sites of ZSM-5 zeolites modified by steaming. J. Catal. 1988, 110, 404–406. [Google Scholar] [CrossRef]
- Derouane, E.G. Shape selectivity in catalysis by zeolites: The nest effect. J. Catal. 1986, 100, 541–544. [Google Scholar] [CrossRef]
- Williams, B.A.; Babitz, S.M.; Miller, J.T.; Snurr, R.Q.; Kung, H.H. The roles of acid strength and pore diffusion in the enhanced cracking activity of steamed Y zeolites. Appl. Catal. A Gen. 1999, 177, 161–175. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Wallenstein, D.; Seese, M.; Zhao, X. A novel selectivity test for the evaluation of FCC catalysts. Appl. Catal. A Gen. 2002, 231, 227–242. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








| Catalyst & Treatment | Total (BET) Surface Area (SA) (m2/g) | Micro-Pore SA (1) (m2/g) | Macro/Meso-Pore & External SA (2) (m2/g) | Micropore Volume (1) (cc/g) | Relative Crystallinity (%) | Brønsted Acidity (μmol Pyridine/g) at 150 °C | Lewis Acidity (μmol Pyridine/g) at 150 °C |
|---|---|---|---|---|---|---|---|
| “Fresh”(Calcined at 732 °C /1 h) | 159 | 122 | 37 | 0.047 | 100 | 15 | 8 |
| Steamed (788 °C/20 h) | 157 | 80 | 77 | 0.032 | 98 | 5 | 3 |
| Steamed (815 °C/20 h) | 150 | 74 | 76 | 0.029 | 92 | 3 | 1.7 |
| Catalyst | Total (BET) Surface Area (m2/g) | Micropore Surface Area (2) (m2/g) | Micropore Volume (2) (cc/g) | Meso/Macropore & External Surface Area (3) (m2/g) | Brønsted Acidity (μmol Pyridine/g) at 150 °C | Lewis Acidity (μmol Pyridine/g) at 150 °C |
|---|---|---|---|---|---|---|
| P/H-ZSM-5 zeolite | 270 | 225 | 0.087 | 45 | 67 | 4 |
| P/H-ZSM-5 zeolite steamed | 304 | 201 | 0.082 | 103 | 25 | 5 |
| SiO2-Al2O3 | 579 | - | - | 579 | N/A | N/A |
| SiO2-Al2O3 steamed | 260 | - | - | 260 | N/A | N/A |
| P/ZSM-5 additive | 159 | 122 | 0.047 | 36 | N/A | N/A |
| P/ZSM-5 additive steamed | 167 | 92 | 0.038 | 75 | N/A | N/A |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusev, A.A.; Psarras, A.C.; Triantafyllidis, K.S.; Lappas, A.A.; Diddams, P.A. Effect of Steam Deactivation Severity of ZSM-5 Additives on LPG Olefins Production in the FCC Process. Molecules 2017, 22, 1784. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22101784
Gusev AA, Psarras AC, Triantafyllidis KS, Lappas AA, Diddams PA. Effect of Steam Deactivation Severity of ZSM-5 Additives on LPG Olefins Production in the FCC Process. Molecules. 2017; 22(10):1784. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22101784
Chicago/Turabian StyleGusev, Andrey A., Antonios C. Psarras, Konstantinos S. Triantafyllidis, Angelos A. Lappas, and Paul A. Diddams. 2017. "Effect of Steam Deactivation Severity of ZSM-5 Additives on LPG Olefins Production in the FCC Process" Molecules 22, no. 10: 1784. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22101784