The Rosiglitazone-Like Effects of Vitexilactone, a Constituent from Vitex trifolia L. in 3T3-L1 Preadipocytes
Abstract
:1. Introduction
2. Results
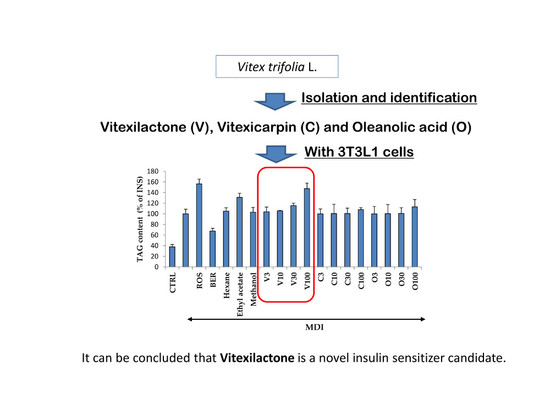
2.1. Yields, Cytotoxicity, and Regulatory Effects on Adipogenesis of the Extracts from V. trifolia
2.2. Isolation of Constituents by Chromatography
2.3. Characterization of the Isolated Compounds
2.4. Cytotoxicity and Regulatory Effects on Adipogenesis of the Compounds Isolated from V. trifolia
2.5. The Effects of 1 on Insulin Receptor Substrate-1 (IRS-1)
2.6. Effect of Vitexilactone on Signal Transduction-Related Proteins
2.7. Effect of Vitexilactone on Sugar/Fat Metabolism-Related Proteins
2.8. Effect of PPARγ Antagonist and MAPKs Inhibitor on Intracellular Lipid Accumulation by MDI Solution with or without ROS or Vitexilactone
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Solvent Fractionation
4.3. Isolation of Active Constituents
4.4. Analytical Instrument of Isolated Components
4.5. Cell Culture
4.6. Cell Toxicity Assay
4.7. Measurement of Intracellular Triglycerol Level
4.8. Detection of Proteins
4.9. Treatment with Specific Inhibitors
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacobson, A.M.; de Groot, M.; Samson, J.A. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care 1994, 17, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef] [PubMed]
- Lo Furno, D.; Graziano, A.C.; Avola, R.; Giuffrida, R.; Perciavalle, V.; Bonina, F.; Mannino, G.; Cardile, V. A Citrus Bergamia Extract Decreases Adipogenesis and Increases Lipolysis by Modulating PPAR Levels in Mesenchymal Stem Cells from Human Adipose Tissue. PPAR Res. 2016, 2016, 4563815. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Miao, J.J.; Sun, X.Y.; Yu, J.Y. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-alpha/gamma and attenuating endoplasmic reticulum stress in rats. J. Ethnopharmacol. 2016, 189, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Gholamhosseinian, A.; Fallah, H. Trigonella foenum-graecum water extract improves insulin sensitivity and stimulates PPAR and gamma gene expression in high fructose-fed insulin-resistant rats. Adv. Biomed. Res. 2016, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, M.; Arumugam, G. Effect of Persea americana (avocado) fruit extract on the level of expression of adiponectin and PPAR-gamma in rats subjected to experimental hyperlipidemia and obesity. J. Complement. Integr. Med. 2014, 11, 107–119. [Google Scholar] [PubMed]
- Mohanbabu, A.V.; Kishore, M.K.; Chandrashekar, B.R.; Pradeepa, H.D.; Christopher, R.; Nandit, P.B. Evaluation of potential antiamnesic activities of aqueous extract of Vitex trifolia leaves against scopolamine induced amnesia and in normal rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Mathankumar, M.; Tamizhselvi, R.; Manickam, V.; Purohit, G. Assessment of Anticarcinogenic Potential of Vitex trifolia and Triticum aestivum Linn by In Vitro Rat Liver Microsomal Degranulation. Toxicol. Int. 2015, 22, 114–118. [Google Scholar] [PubMed]
- Wu, J.; Zhou, T.; Zhang, S.W.; Zhang, X.H.; Xuan, L.J. Cytotoxic terpenoids from the fruits of Vitex trifolia L. Planta Med. 2009, 75, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Cui, C.B.; Cai, B.; Yao, X.S. Labdane-type diterpenes as new cell cycle inhibitors and apoptosis inducers from Vitex trifolia L. J. Asian Nat. Prod. Res. 2005, 7, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Cui, C.B.; Cai, B.; Wang, H.Y.; Yao, X.S. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J. Asian Nat. Prod. Res. 2005, 7, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Adib-Conquy, M.; Coste, A.; Kumar-Roine, S.; Pipy, B.; Laurent, D.; Pauillac, S. Aqueous extract of Vitex trifolia L. (Labiatae) inhibits LPS-dependent regulation of inflammatory mediators in RAW 264.7 macrophages through inhibition of Nuclear Factor kappa B translocation and expression. J. Ethnopharmacol. 2012, 143, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Kumar-Roine, S.; Darius, H.T.; Chinain, M.; Laurent, D.; Pauillac, S. Characterisation of the anti-inflammatory potential of Vitex trifolia L. (Labiatae), a multipurpose plant of the Pacific traditional medicine. J. Ethnopharmacol. 2009, 126, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kannathasan, K.; Senthilkumar, A.; Venkatesalu, V. Mosquito larvicidal activity of methyl-p-hydroxybenzoate isolated from the leaves of Vitex trifolia Linn. Acta Trop. 2011, 120, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.K.; Vidya, S.M.; Krishna, V.; Mankani, K.L.; Singh, S.D.; Manohara, Y.N. Comparative evaluation of wound healing potency of Vitex trifolia L. and Vitex altissima L. Phytother. Res. 2007, 21, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Geetha, V.; Doss, A.; Doss, A.P. Antimicrobial potential of Vitex trifolia Linn. Anc. Sci. Life 2004, 23, 30–32. [Google Scholar] [PubMed]
- Hossain, M.M.; Paul, N.; Sohrab, M.H.; Rahman, E.; Rashid, M.A. Antibacterial activity of Vitex trifolia. Fitoterapia 2001, 72, 695–697. [Google Scholar] [CrossRef]
- Hernandez, M.M.; Heraso, C.; Villarreal, M.L.; Vargas-Arispuro, I.; Aranda, E. Biological activities of crude plant extracts from Vitex trifolia L. (Verbenaceae). J. Ethnopharmacol. 1999, 67, 37–44. [Google Scholar] [CrossRef]
- Zheng, C.J.; Zhu, J.Y.; Yu, W.; Ma, X.Q.; Rahman, K.; Qin, L.P. Labdane-type diterpenoids from the fruits of Vitex trifolia. J. Nat. Prod. 2013, 76, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Zhang, X.M.; Zhou, J.; Qiu, S.X.; Chen, J.J. One new dihydrobenzofuran lignan from Vitex trifolia. J. Asian Nat. Prod. Res. 2008, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Yamamoto, M.; Yanaka, T.; Ito, Y.; Nohara, T. Ten new labdane-type diterpenes from the fruit of Vitex rotundifolia. Chem. Pharm. Bull. 2001, 49, 82–86. [Google Scholar] [CrossRef] [PubMed]
- You, K.M.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Vitexicarpin, a flavonoid from the fruits of Vitex rotundifolia, inhibits mouse lymphocyte proliferation and growth of cell lines in vitro. Planta Med. 1998, 64, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhu, L.; Li, J.; Qin, R.; Liu, C.; Chen, Y.; Yang, G. Synthesis and structure elucidation of five new conjugates of oleanolic acid derivatives and chalcones using 1D and 2D NMR spectroscopy. Magn. Reson. Chem. 2012, 50, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Somwar, R.; Bilan, P.J.; Liu, Z.; Jin, J.; Woodgett, J.R.; Klip, A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol. Cell. Biol. 1999, 19, 4008–4018. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shen, N.; Zhang, M.L.; Pan, F.Y.; Wang, C.; Jia, W.P.; Liu, C.; Gao, Q.; Gao, X.; Xue, B.; et al. Egr-1 decreases adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal balance in mice. EMBO J. 2011, 30, 3754–3765. [Google Scholar] [CrossRef] [PubMed]
- Tanti, J.F.; Gual, P.; Gremeaux, T.; Gonzalez, T.; Barres, R.; Le Marchand-Brustel, Y. Alteration in insulin action: Role of IRS-1 serine phosphorylation in the retroregulation of insulin signalling. Ann. Endocrinol. 2004, 65, 43–48. [Google Scholar] [CrossRef]
- Li, W.; Liang, X.; Zeng, Z.; Yu, K.; Zhan, S.; Su, Q.; Yan, Y.; Mansai, H.; Qiao, W.; Yang, Q.; et al. Simvastatin inhibits glucose uptake activity and GLUT4 translocation through suppression of the IR/IRS-1/Akt signaling in C2C12 myotubes. Biomed. Pharmacother. 2016, 83, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S.; Rubinstein, M. FABP4 attenuates PPARgamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes 2014, 63, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M.; Kloting, N.; Wueest, S.; Schoenle, E.J.; Schon, M.R.; Dietrich, A.; Fasshauer, M.; Stumvoll, M.; Konrad, D. Fas and FasL expression in human adipose tissue is related to obesity, insulin resistance, and type 2 diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E36–E44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.P.; She, M.H.; He, P.P.; Chen, W.J.; Laudon, M.; Xu, X.X.; Yin, W.D. Piromelatine decreases triglyceride accumulation in insulin resistant 3T3-L1 adipocytes: Role of ATGL and HSL. Biochimie 2013, 95, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
- Jocken, J.W.; Langin, D.; Smit, E.; Saris, W.H.; Valle, C.; Hul, G.B.; Holm, C.; Arner, P.; Blaak, E.E. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J. Clin. Endocrinol. Metab. 2007, 92, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Izawa, T.; Ogasawara, J.E.; Sakurai, T.; Nomura, S.; Kizaki, T.; Ohno, H.; Komabayashi, T. Effect of exercise training on adipocyte-size-dependent expression of leptin and adiponectin. Life Sci. 2010, 86, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.J.; Hook, J.M.; Al-Deen, T.S.; Hibbert, D.B. Quantitative nuclear magnetic resonance (QNMR) spectroscopy for assessing the purity of technical grade agrochemicals: 2,4-dichlorophenoxyacetic acid (2,4-D) and sodium 2,2-dichloropropionate (Dalapon sodium). J. Agric. Food Chem. 2002, 50, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, K.; Shibano, M. Avicularin, a plant flavonoid, suppresses lipid accumulation through repression of C/EBPalpha-activated GLUT4-mediated glucose uptake in 3T3-L1 cells. J. Agric. Food Chem. 2013, 61, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Nishina, A.; Kimura, H.; Sekiguchi, A.; Fukumoto, R.H.; Nakajima, S.; Furukawa, S. Lysophosphatidylethanolamine in Grifola frondosa as a neurotrophic activator via activation of MAPK. J. Lipid Res. 2006, 47, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available. |








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishina, A.; Itagaki, M.; Sato, D.; Kimura, H.; Hirai, Y.; Phay, N.; Makishima, M. The Rosiglitazone-Like Effects of Vitexilactone, a Constituent from Vitex trifolia L. in 3T3-L1 Preadipocytes. Molecules 2017, 22, 2030. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22112030
Nishina A, Itagaki M, Sato D, Kimura H, Hirai Y, Phay N, Makishima M. The Rosiglitazone-Like Effects of Vitexilactone, a Constituent from Vitex trifolia L. in 3T3-L1 Preadipocytes. Molecules. 2017; 22(11):2030. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22112030
Chicago/Turabian StyleNishina, Atsuyoshi, Masaya Itagaki, Daisuke Sato, Hirokazu Kimura, Yasuaki Hirai, Nyunt Phay, and Makoto Makishima. 2017. "The Rosiglitazone-Like Effects of Vitexilactone, a Constituent from Vitex trifolia L. in 3T3-L1 Preadipocytes" Molecules 22, no. 11: 2030. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22112030