A New Synthetic Route to Polyhydrogenated Pyrrolo[3,4-b]pyrroles by the Domino Reaction of 3-Bromopyrrole-2,5-Diones with Aminocrotonic Acid Esters
Abstract
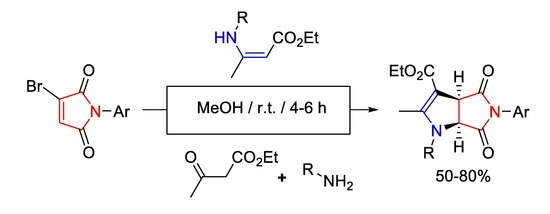
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Procedure for the Reaction of Bromomaleimides (1) with Aminocrotonates (3) and Characterization Data of Pyrrolo[3,4-b]pyrroles (8a–f)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krzeszewski, M.; Gryko, D.; Gryko, D.T. The tetraarylpyrrolo[3,2-b]pyrroles-from serendipitous discovery to promising heterocyclic optoelectronic materials. Acc. Chem. Res. 2017, 50, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Molecular design of photovoltaic materials for polymer solar sells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, T.K.; Patri, M.; Mishra, S.P. Highly sensitive colorimetric and fluorometric anion sensors based on mono and di-calix[4]pyrrole substituted diketopyrrolopyrroles. Sens. Actuators B Chem. 2016, 225, 428–435. [Google Scholar] [CrossRef]
- Wiktorowski, S.; Christelle, R.; Winterhalder, M.; Daltrozzo, E.; Zumbusch, A. Water-soluble pyrrolopyrrole cyanine (PPCy) NIR fluorophores. Chem. Commun. 2014, 50, 4755–4758. [Google Scholar] [CrossRef] [PubMed]
- Vala, M.; Vyňuchal, J.; Toman, P.; Weiter, M.; Luňák, S., Jr. Novel, soluble diphenyl-diketo-pyrrolopyrroles: Experimental and theoretical study. Dyes Pigments 2010, 84, 176–182. [Google Scholar] [CrossRef]
- Mizuguchi, J.; Shikamori, H. Spectral and Crystallographic Coincidence in a Mixed Crystal of Two Components and a Crystal of Their Hybrid Component in Pyrrolopyrrole Pigments. J. Phys. Chem. B 2004, 108, 2154–2161. [Google Scholar] [CrossRef]
- Cordes, J.; Harms, K.; Koert, U. Synthesis of the Isoquinocycline-Pyrrolopyrrole Substructure. Org. Lett. 2010, 12, 3808–3811. [Google Scholar] [CrossRef] [PubMed]
- Muchowski, J.M.; Unger, S.H.; Ackrell, J.; Cheung, P.; Cooper, G.F.; Cook, J.; Gallegra, P.; Halpern, O.; Koehler, R.; Kluge, A.F. Synthesis and Antiinflammatory and Analgesic Activity of 5-Aroyl-1,2-dihydro-3H-pyrrolo[1,2-a]pyrrole-1-carboxylic Acids and Related Compounds. J. Med. Chem. 1989, 32, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Che, X.; Bao, G.; Wang, N.; Peng, L.; Barnash, K.D.; Frye, S.V.; James, L.I.; Bai, X. Design, synthesis, and protein methyltransferase activity of a unique set of constrained amine containing compounds. Bioorg. Med. Chem. Lett. 2016, 26, 4436–4440. [Google Scholar] [CrossRef] [PubMed]
- Trunkfield, A.E.; Gurcha, S.S.; Gurdyal, S.B.; Timothy, D.H. Inhibition of Escherichia coli glycosyltransferase MurG and Mycobacterium tuberculosis Gal transferase by uridine-linked transition state mimics. Bioorg. Med. Chem. 2010, 18, 2651–2663. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.G.; Beer, M.S.; Stanton, J.A.; Sohal, B.; Castro, J.L. 2,7-Diazabicyclo[3.3.0]octanes as novel h5-HT1D receptor agonists. Bioorg. Med. Chem. Lett. 1999, 9, 2491–2496. [Google Scholar] [CrossRef]
- Huck, B.R.; Llamas, L.; Robarge, M.J.; Dent, T.C.; Song, J.; Hodnick, W.F.; Crumrine, C.; Stricker-Krongrad, A.; Harrington, J. The identification of pyrimidine-diazabicyclo[3.3.0]octane derivatives as 5-HT2C receptor agonists. Bioorg. Med. Chem. Lett. 2006, 16, 2891–2894. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, A.A.; Ivashchenko, A.V.; Tkachenko, S.E.; Okun, M.; Savchuk, N.F. Ligands for 5-HT6, Pharmaceutical Composition, Method for Their Production and Use Thereof. U.S. Patent Application No. 2011/0046368 A1, 24 February 2011. [Google Scholar]
- Chang, L.L.; Yang, G.X.; McCauley, E.; Mumford, R.A.; Schmidt, J.A.; Hagmann, W.K. Constraining the amide bond in N-Sulfonylated dipeptide VLA-4 antagonists. Bioorg. Med. Chem. Lett. 2008, 18, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chu, D.T.W.; Cooper, C.S.; Li, Q.; Fung, A.K.L.; Wang, S.; Fung, S.W.; Shen, L.L.; Flamm, R.K.; Nilius, A.M.; et al. Synthesis and Antimicrobial Activity of 4H-4-Oxoquinolizine Derivatives: Consequences of Structural Modification at the C-8 Position. J. Med. Chem. 1999, 42, 4202–4213. [Google Scholar] [CrossRef] [PubMed]
- Schenke, T.; Petersen, U. Preparation of 2,7-Diazabicyclo[3,3,0]octanes. U.S. Patent 5,071,999, 10 December 1991. [Google Scholar]
- Pedrosa, R.; Andrés, C.; Andrés, L.; Nieto, J. A Novel Synthesis of Enantiopure Octahydropyrrolo[3,4-b]pyrroles by Intramolecular [3 + 2] Dipolar Cycloaddition on Chiral Perhydro-1,3-benzoxazines. Org. Lett. 2002, 4, 2513–2516. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.; Andrés, C.; Nieto, J.; Pérez-Cuadrado, C.; San Francisco, I. An Efficient and Diastereoselective Intramolecular 1,3-Dipolar Cycloaddition of Cyclic Azomethine Ylides and Nitrones. Eur. J. Org. Chem. 2006, 14, 3259–3265. [Google Scholar] [CrossRef]
- Poornachandran, M.; Raghunathan, R. A novel entry into 1-methyl- and 1-aryl-octahydropyrrolo[3,4-b]pyrroles and their N-1–C-2 fused derivatives: Stereoselective synthesis via an intramolecular azomethine ylide cycloaddition reaction. Tetrahedron Lett. 2005, 46, 7197–7200. [Google Scholar] [CrossRef]
- Poornachandran, M.; Raghunathan, R. Synthesis of pyrrolo[3,4-b]pyrroles and perhydrothiazolo-[3’,4’-2,3]pyrrolo[4,5-c]pyrroles. Tetrahedron 2008, 64, 6461–6474. [Google Scholar] [CrossRef]
- Poornachandran, M.; Raghunathan, R. Facile Synthesis of cis-Fused 1-Benzyl/-H-5-arylsulfonyl Pyrrolo[3,4-b]pyrroles. Synth. Commun. 2009, 39, 917–926. [Google Scholar] [CrossRef]
- Morin, M.S.T.; Aly, S.; Arndtsen, B.A. Phosphonite mediated 1,3-dipolar cycloaddition: A route to polycyclic 2-pyrrolines from imines, acid chlorides and alkenes. Chem. Commun. 2013, 49, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Kim, K.-J.; Hong, Y.-T. Synthesis of α-Methylene-γ-butyrolactones: Ru-Catalyzed Cyclocarbonylation of Allenyl Aldehydes and Allenyl Ketones. Angew. Chem. Int. Ed. 2002, 41, 1584–1586. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, E.S.; Yu, C.M. A Cyclocarbonylation for the Synthesis of Bicyclic 3-Methylene-1-phthalimidoylbutyrolactams from Allene-hydrazones Mediated by Mo(CO)6. Synlett 2007, 15, 2439–2441. [Google Scholar] [CrossRef]
- Lee, J.W.; Son, H.J.; Lee, J.H.; Yoon, G.J.; Park, M.H. Synthesis of 2,7-Diazabicyclo[3.3.0]octane Derivatives via Intramolecular Cyclization Reaction. Synth. Commun. 1996, 26, 89–94. [Google Scholar] [CrossRef]
- Lee, J.W.; Son, H.J.; Jung, Y.E.; Lee, J.H. Synthesis of 2,7-Diazabicyclo[3.3.0]octane and 2,7-Diazabicyclo[3.3.0]oct-4-ene Derivatives via Cyclization Reaction and Julia Reaction. Synth. Commun. 1996, 26, 1499–1505. [Google Scholar] [CrossRef]
- Attanasi, J.A.; Bianchi, L.; De Crescentini, L.; Favi, G.; Mantellini, F. Easy One-Pot Synthesis of Fused Heterocycles from 1,2-Diaza-1,3-dienes. Eur. J. Org. Chem. 2011, 2924–2927. [Google Scholar] [CrossRef]
- Guillaume, J.; Lubell, W.D. Synthesis of Fused Heteroarylprolines and Pyrrolopyrroles. J. Org. Chem. 2004, 69, 4656–4662. [Google Scholar]
- Lee, S.; Chataigner, I.; Piettre, S.R. Facile Dearomatization of Nitrobenzene Derivatives and Other Nitroarenes with N-Benzyl Azomethine Ylide. Angew. Chem. Int. Ed. 2011, 50, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, C.; You, S.-L. Catalytic C6 Functionalization of 2,3-Disubstituted Indoles by Scandium Triflate. J. Org. Chem. 2014, 79, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, H.-X.; Chen, F.; Zhang, Z.-B.; Zou, Y.; Chen, C.; Song, X.-J.; Tian, F.; Peng, L.; Wang, L.-X. Organocatalytic Asymmetric Annulation between Hydroxymaleimides and Nitrosoarenes: Stereoselective Preparation of Chiral Quaternary N-Hydroxyindolines. Org. Lett. 2017, 19, 2805–2808. [Google Scholar] [CrossRef] [PubMed]
- Rostovskii, N.V.; Novikov, M.S.; Khlebnikov, A.F.; Korneev, S.M.; Yufit, D.S. Cu(II)-catalyzed domino reaction of 2H-azirines with diazotetramic and diazotetronic acids. Synthesis of 2-substituted 2H-1,2,3-triazoles. Org. Biomol. Chem. 2013, 11, 5535–5545. [Google Scholar] [CrossRef] [PubMed]
- Rostovskii, N.V.; Sakharov, P.A.; Novikov, M.S.; Khlebnikov, A.F.; Starova, G.L. Cu(I)–NHC-Catalyzed (2 + 3)-Annulation of Tetramic Acids with 2H-Azirines: Stereoselective Synthesis of Functionalized Hexahydropyrrolo[3,4-b]pyrroles. Org. Lett. 2015, 17, 4148–4151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-N.; Chen, L.-L.; Zhou, R.-X.; Li, B.; Shao, Z.-Y.; Zhao, S.-Y. Copper-Catalyzed Oxidative Cyclization of Maleimides with Amines and Alkyne Esters: Direct Access to Fully Substituted Dihydropyrroles and Pyrrole Derivatives. Org. Lett. 2017, 19, 6044–6047. [Google Scholar] [CrossRef] [PubMed]
- Vandyshev, D.Y.; Shikhaliev, K.S.; Potapov, A.Y.; Krysin, M.Y. Condensation of 1,2-diamino-4-phenylimidazole and N-arylmaleimides with the formation of new tetrahydroimidazo[1,5-b]pyridazines. Chem. Heterocycl. Comp. 2015, 51, 829–833. [Google Scholar] [CrossRef]
- Vandyshev, D.Y.; Shikhaliev, K.S.; Kokonova, A.V.; Potapov, A.Y.; Kolpakova, M.G.; Sabynin, A.L.; Zubkov, F.I. A novel method for the synthesis of pyrimido[1,2-a]benzimidazoles. Chem. Heterocycl. Comp. 2016, 52, 493–497. [Google Scholar] [CrossRef]
- Rudenko, R.V.; Komykhov, S.A.; Desenko, S.M.; Sen’ko, Y.V.; Shishkin, O.V.; Konovalova, I.S.; Shishkina, S.V.; Chebanov, V.A. A Comprehensive Study of the Heterocyclizations of N-Arylmaleimides and 6-Aminouracils. Synthesis 2011, 3161–3167. [Google Scholar] [CrossRef]
- Havrylyuk, D.; Zimenkovsky, B.; Lesyk, R. Synthesis and Anticancer Activity of Novel Nonfused Bicyclic Thiazolidinone Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 638–650. [Google Scholar] [CrossRef]
- Lesyk, R.; Vladzimirska, O.; Holota, S.; Zaprutko, L.; Gzella, A. New 5-substituted thiazolo[3,2-b][1,2,4]triazol-6-ones: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2007, 42, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.R.; DeWitt Blanton, C. Reaction of Maleimides and Ethyl 3-Aminocrotonates, a Reinvesiation Leading to an Improved Synthesis of Pyrrolo[3,4-c] pyridines. J. Org. Chem. 1982, 47, 502–508. [Google Scholar] [CrossRef]
- Banwella, M.G.; Jonesa, M.T.; Loonga, D.T.; Luptona, D.W.; Pinkertona, D.M.; Rayb, J.K.; Willisa, A.S. A Pd[0]-catalyzed Ullmann cross-coupling/reductive cyclization approach to C-3 mono-alkylated oxindoles and related compounds. Tetrahedron 2010, 66, 9252–9262. [Google Scholar] [CrossRef]
- Kuckländer, U.; Hühnermann, W. Beobachtungen zum Mechanismus der Nenitzescu-Reaktion Synthese von 6-Hydroxy-indol-Derivaten. Arch. Pharm. 1979, 312, 515–526. [Google Scholar] [CrossRef]
- George, R.; Allen, G.R., Jr.; Pidacks, C.; Weiss, M.J. The Mitomycin Antibiotics. Synthetic Studies. XIV.1 the Nenitzescu Indole Synthesis. Formation of Isomeric Indoles and Reaction Mechanism. J. Am. Chem. Soc. 1966, 88, 2536–2544. [Google Scholar]
- Zhang, Y.; Chen, F.; Yang, Y.; Tang, C.-Z.; Tian, F.; Peng, L.; Wang, L.-X. An unexpected metal-free DMAP catalyzed Michael addition–elimination domino reaction between 2-naphthols and bromomaleimides for the effective construction of 3-arylmaleimides. Tetrahedron Lett. 2016, 57, 1261–1264. [Google Scholar] [CrossRef]
- Estevez, V.; Villacampa, M.; Menéndez, J.C. Recent advances in the synthesis of pyrroles by multicomponent reactions. Chem. Soc. Rev. 2014, 43, 4633–4657. [Google Scholar] [CrossRef] [PubMed]
- Moss, T.A.; Nowak, T. Synthesis of 2,3-dicarbonylated pyrroles and furans via the three-component Hantzsch reaction. Tetrahedron Lett. 2012, 53, 3056–3060. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Yang, W.; Zhuang, J.; Wang, W. A mild and selective protecting and reversed modification of thiols. Tetrahedron Lett. 2016, 57, 2660–2663. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 8 are available from the authors. |






| Entry | Bromomaleimide, Ar | Aminocrotonate, R | Product | Time (h) | Yields 1 (%) |
|---|---|---|---|---|---|
| 1 | Ph (1a) | Ph (2a) | 8a | 5 | 46/53 |
| 2 | Ph (1a) | 4-MeOC6H4 (2b) | 8b | 5 | 77/69 |
| 3 | 4-EtC6H4 (1b) | PhCH2 (2c) | 8c | 4 | 82/74 |
| 4 | 4-EtOC6H4 (1c) | PhCH2 (2c) | 8d | 6 | 73/70 |
| 5 | 3,4-Cl2C6H3 (1d) | Ph (2a) | 8e | 6 | 70/64 |
| 6 | 3,4-Cl2C6H3 (1d) | PhCH2 (2c) | 8f | 6 | 69/61 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shikhaliev, K.; Sabynin, A.; Sekirin, V.; Krysin, M.; Zubkov, F.; Yankina, K. A New Synthetic Route to Polyhydrogenated Pyrrolo[3,4-b]pyrroles by the Domino Reaction of 3-Bromopyrrole-2,5-Diones with Aminocrotonic Acid Esters. Molecules 2017, 22, 2035. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22112035
Shikhaliev K, Sabynin A, Sekirin V, Krysin M, Zubkov F, Yankina K. A New Synthetic Route to Polyhydrogenated Pyrrolo[3,4-b]pyrroles by the Domino Reaction of 3-Bromopyrrole-2,5-Diones with Aminocrotonic Acid Esters. Molecules. 2017; 22(11):2035. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22112035
Chicago/Turabian StyleShikhaliev, Khidmet, Artem Sabynin, Valeri Sekirin, Michael Krysin, Fedor Zubkov, and Kristina Yankina. 2017. "A New Synthetic Route to Polyhydrogenated Pyrrolo[3,4-b]pyrroles by the Domino Reaction of 3-Bromopyrrole-2,5-Diones with Aminocrotonic Acid Esters" Molecules 22, no. 11: 2035. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22112035