Studies on the Inclusion Complexes of Daidzein with β-Cyclodextrin and Derivatives
Abstract
:1. Introduction
2. Results and Discussion
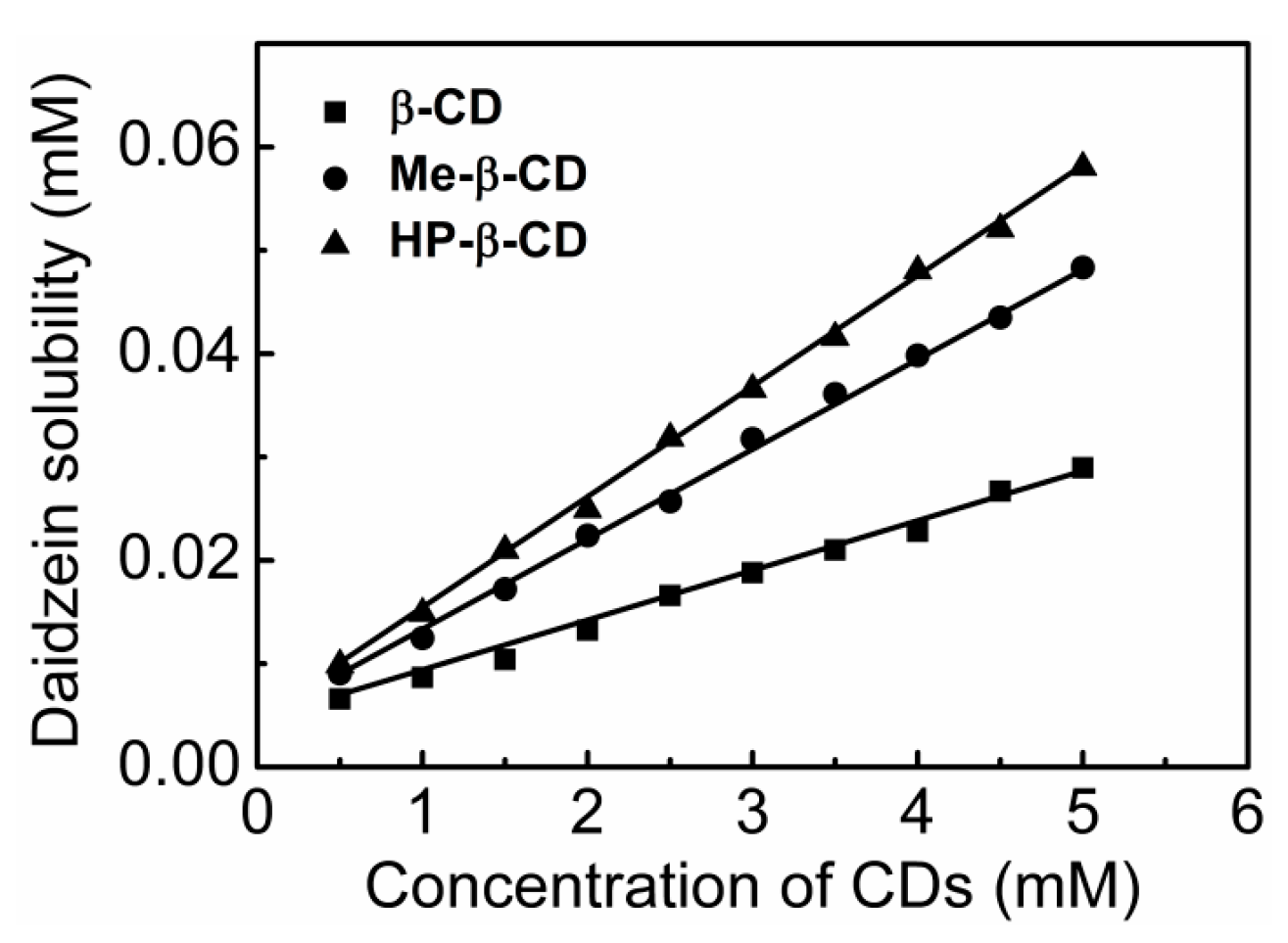
2.1. Phase-Solubility Study
2.2. XRD Studies
2.3. Thermal Analysis
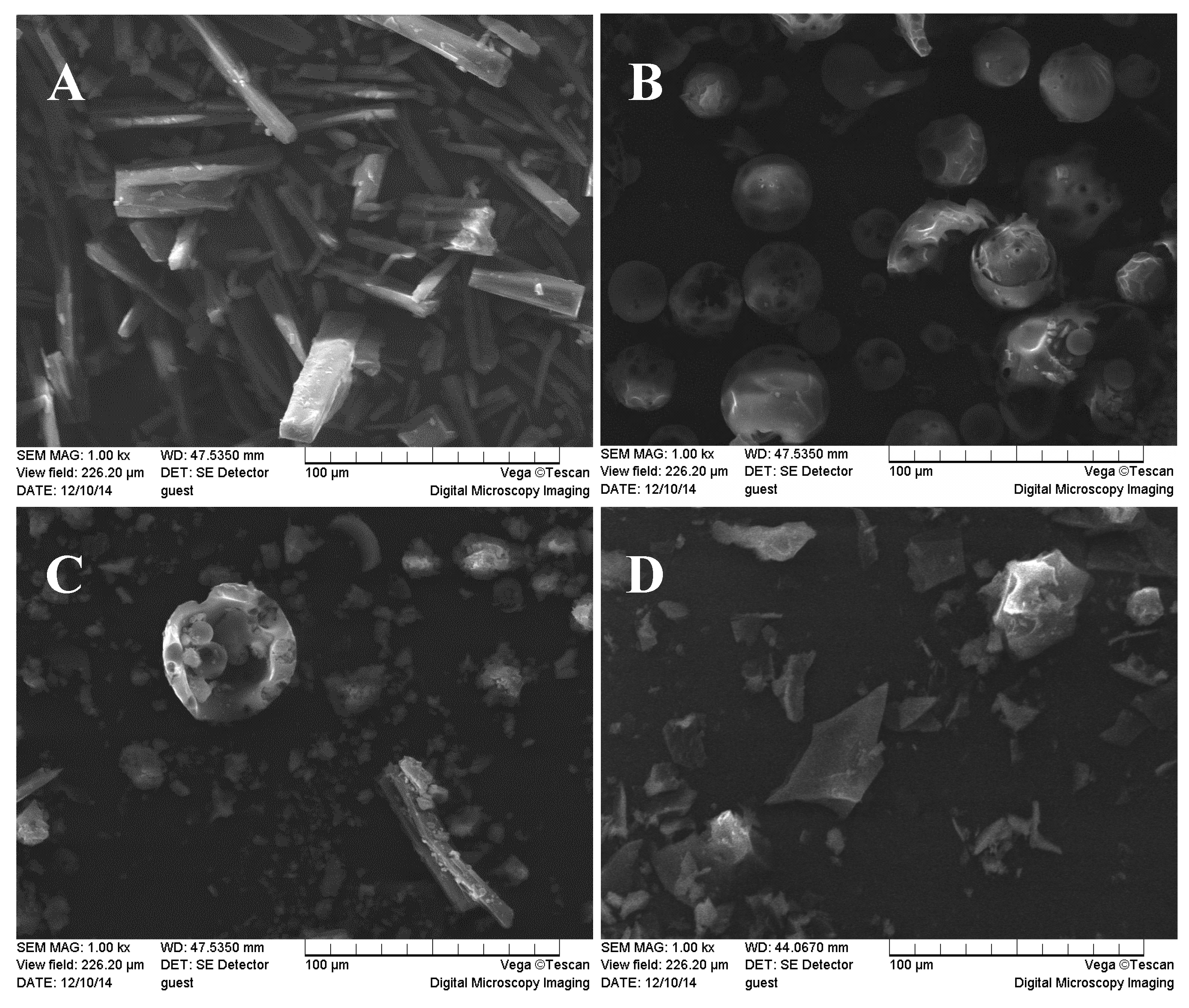
2.4. SEM Studies
2.5. 1H-NMR and 2D NMR
2.6. Antioxidant Activity of Daidzein in Free and Complex Form
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Daidzein/β-CD, Daidzein/Me-β-CD and Daidzein/HP-β-CD Inclusion Complexes
3.2.2. Preparation of Daidzein/β-CD, Daidzein/Me-β-CD and Daidzein/HP-β-CD Physical Mixture
3.2.3. Phase-Solubility Study
3.2.4. Stoichiometry Determination: Job’s Method
3.2.5. Powder X-ray Diffraction (XRD)
3.2.6. Thermal Analyses
3.2.7. Scanning Electron Microscopy (SEM)
3.2.8. 1H-NMR and 2D NMR
3.2.9. DPPH Radical-Scavenging Capacity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Setchell, K.D.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758S–767S. [Google Scholar] [PubMed]
- Tikkanen, M.J.; Wahala, K.; Ojala, S.; Vihma, V.; Adlercreutz, H. Effect of soybean phytoestrogen intake on low density lipoprotein oxidation resistance. Proc. Natl. Acad. Sci. USA 1998, 95, 3106–3110. [Google Scholar] [CrossRef] [PubMed]
- Kerry, N.; Abbey, M. The isoflavone genistein inhibits copper and peroxyl radical mediated low density lipoprotein oxidation in vitro. Atherosclerosis 1998, 140, 341–347. [Google Scholar] [CrossRef]
- Wada, K.; Nakamura, K.; Tamai, Y.; Tsuji, M.; Kawachi, T.; Hori, A.; Takeyama, N.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; et al. Soy isoflavone intake and breast cancer risk in Japan: From the Takayama study. Int. J. Cancer 2013, 133, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Su, S.J.; Traiming, Y.; Lei, H.Y.; Nanhaw, C. The potential of soybean foods as a chemoprevention approach for human urinary tract cancer. Clin. Cancer Res. 2000, 6, 230–236. [Google Scholar] [PubMed]
- Chacko, B.K.; Chandler, R.T.; D’Alessandro, T.L.; Mundhekar, A.; Khoo, N.K.; Botting, N.; Barnes, S.; Patel, P.R. Anti-inflammatory effects of isoflavones are dependent on flow and human endothelial cell ppargamma. J. Nutr. 2007, 137, 351–356. [Google Scholar] [PubMed]
- Widyarini, S.; Spinks, N.; Husband, A.J.; Reeve, V.E. Isoflavonoid compounds from red clover (Trifolium pratense) protect from inflammation and immune suppression induced by UV radiation. Photochem. Photobiol. 2001, 74, 465–470. [Google Scholar] [CrossRef]
- Marotta, F.; Mao, G.S.; Liu, T.; Chui, D.H.; Lorenzetti, A.; Xiao, Y. Marandola, P. Anti-inflammatory and neuroprotective effect of a phytoestrogen compound on rat microglia. Ann. N. Y. Acad. Sci. 2006, 1089, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T. Soy, soy phytoestrogens and cardiovascular disease. J. Nutr. 2002, 132, 566S–569S. [Google Scholar] [PubMed]
- Barnes, S. Evolution of the health benefits of soy isoflavones. Proc. Soc. Exp. Biol. Med. 1998, 217, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.R.; Hung, C.F.; Lin, Y.K.; Fang, J.Y. In vitro and in vivo evaluation of topical delivery and potential dermal use of soy isoflavones genistein and daidzein. Int. J. Pharm. 2008, 364, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.M.; Yang, L.G.; Mao, P.; Yuan, J.W.; Deng, Y.X.; Qu, L.B. Inclusion complexes of phosphorylated daidzein derivatives with β-cyclodextrin: Preparation and inclusion behavior study. Spectrochim. Acta Part A 2012, 85, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Hamada, H. Synthesis of β-Maltooligosaccharides of Glycitein and Daidzein and their anti-oxidant and anti-allergic activities. Molecules 2010, 15, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rajewski, R.A. Cyclodextrins: Their future in drug formulation and delivery. Pharm. Res. 1997, 14, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Tablet, C.; Minea, L.; Dumitrache, L.; Hillebrand, M. Experimental and theoretical study of the inclusion complexes of 3-carboxycoumarin acid with β- and 2-hydroxypropyl-β-cyclodextrins. Spectrochim. Acta Part A 2012, 92, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Ma, S.X.; Zhou, S.Y.; Chen, W.; Yuan, M.W.; Yin, Y.Q.; Yang, X.D. Preparation and characterization of inclusion complexes of naringenin with β-cyclodextrin or its derivative. Carbohydr. Polym. 2013, 98, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.F.; Li, K.; Huang, R.; He, G.J.; Zhang, J.Q.; Zhu, L.; Yang, Q.Y.; Jiang, K.M.; Jin, Y.; Lin, J. Investigation of inclusion complex of epothilone A with cyclodextrins. Carbohydr. Polym. 2014, 102, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Blanch, G.P.; Ruiz del Castillo, M.L.; Caja, M.M.; Perez-Mendez, M.; Sanchez-Cortes, S. Stabilization of all-trans-lycopene from tomato by encapsulation using cyclodextrins. Food Chem. 2007, 105, 1335–1341. [Google Scholar] [CrossRef]
- Gallegoyerga, L.; Lomazzi, M.; Sansone, F.; Ortiz, M.C.; Casnati, A.; García Fernández, J.M. Glycoligand-targeted core-shell nanospheres with tunable drug release profiles from calixarene-cyclodextrin heterodimers. Chem. Commun. 2014, 50, 7440–7443. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Y.; Li, C.; Cheng, S.X.; Zhuo, R.X.; Zhang, X.Z. Cyclodextrin-responsive micelles based on poly(ethylene glycol)-polypeptide hybrid copolymers as drug carriers. ACS Macro Lett. 2013, 2, 201–205. [Google Scholar] [CrossRef]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Valle, E.M.M.D. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Stancanelli, R.; Mazzaglia, A.; Tommasini, S.; Calabrò, M.L.; Villari, V.; Guardo, M.; Ficarra, P.; Ficarra, R. The enhancement of isoflavones water solubility by complexation with modified cyclodextrins: A spectroscopic investigation with implications in the pharmaceutical analysis. J. Pharm. Biomed. Anal. 2007, 44, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Yatsua, F.K.J.; Koestera, L.S.; Lula, I.; Passosb, J.J.; Sinisterra, R.; Bassania, V.L. Multiple complexation of cyclodextrin with soy isoflavones present in an enriched fraction. Carbohydr. Polym. 2013, 98, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Nguyen, T.A.; Liu, B.G.; Zhao, J.; Thomas, D.S.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.S.; Xie, Y.; Hong, C.; Li, G.W.; Shen, H.Y.; Ji, G. Development of a myricetin/hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization, and evaluation. Carbohydr. Polym. 2014, 110, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Antioxidant vitamin E/cyclodextrin inclusion complex electrospun nanofibers: Enhanced water solubility, prolonged shelf life, and photostability of vitamin E. J. Agric. Food Chem. 2017, 65, 5404–5412. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kringel, D.H.; Antunes, M.D.; Klein, B.; Crizel, R.L.; Wagner, R.; de Oliveira, R.P.; Dias, A.R.G.; Zavareze, E.D.R. Production, characterization, and stability of orange or eucalyptus essential oil/β-Cyclodextrin inclusion complex. J. Food Sci. 2017, 82, 2598–2605. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Q.; Zhang, J.; Zhou, Y.; Bei, W.Y.; Li, Y.; Yuan, Q.P.; Liang, H. Characterization of glabridin/hydroxypropyl-β-cyclodextrin inclusion complex with robust solubility and enhanced bioactivity. Carbohydr. Polym. 2017, 159, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Sun, H.F.; Bano, S.; Zhao, Y.Y.; Xu, X.Q.; Tang, J. Preparation, characterization, and in vitro anti-inflammatory evaluation of novel water soluble kamebakaurin/hydroxypropyl-β-cyclodextrin inclusion complex. J. Mol. Struct. 2017, 1130, 319–326. [Google Scholar] [CrossRef]
- Borghetti, G.B.; Pinto, A.P.; Lula, I.S.; Sinisterra, R.D.; Teixeira, H.F.; Bassani, V.L. Daidzein/cyclodextrin/hydrophilic polymer ternary systems. Drug Dev. Ind. Pharm. 2011, 37, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, M. Characterizing the natural canthaxanthin/2-hydroxypropyl-β-cyclodextrin inclusion complex. Carbohydr. Polym. 2014, 101, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Jullian, C.; Orosteguis, T.; Pérez-Cruz, F.; Sánchez, P.; Mendizabal, F.; Olea-Azar, C. Complexation of morin with three kinds of cyclodextrin: A thermodynamic and reactivity study. Spectrochim. Acta Part A 2008, 71, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Strazisar, M.; Andrensek, S.; Smidovnik, A. Effect of β-cyclodextrin on antioxidant activity of coumaric acids. Food Chem. 2008, 110, 636–642. [Google Scholar] [CrossRef]
- Liu, M.; Dong, L.N.; Chen, A.J.; Zhang, Y.; Sun, D.Z.; Wang, X.; Wang, B.Q. Inclusion complexes of quercetin with three β-cyclodextrins derivatives at physiological pH: Spectroscopic study and antioxidant activity. Spectrochim. Acta Part A 2013, 115, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Jullian, C.; Cifuentes, C.; Alfaro, M.; Miranda, S.; Barriga, G.; Olea-Azar, C. Spectroscopic characterization of the inclusion complexes of luteolin with native and derivatized β-cyclodextrin. Bioorg. Med. Chem. 2010, 18, 5025–5031. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.S.; Li, B.S.; Zeng, Q.X.; Liu, H.X. Antioxidant and free radical scavenging activities of pigments extracted from molasses alcohol waste water. Food Chem. 2008, 107, 1198–1204. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yuan, L.; Chen, Y.; Zhou, W.; Wang, X. Studies on the Inclusion Complexes of Daidzein with β-Cyclodextrin and Derivatives. Molecules 2017, 22, 2183. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122183
Li S, Yuan L, Chen Y, Zhou W, Wang X. Studies on the Inclusion Complexes of Daidzein with β-Cyclodextrin and Derivatives. Molecules. 2017; 22(12):2183. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122183
Chicago/Turabian StyleLi, Shujing, Li Yuan, Yong Chen, Wei Zhou, and Xinrui Wang. 2017. "Studies on the Inclusion Complexes of Daidzein with β-Cyclodextrin and Derivatives" Molecules 22, no. 12: 2183. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122183



