2.1. Catalysts Characterisation
The X-ray diffraction patterns of the studied H-β samples are shown in
Figure 1. The characteristic sharp and intensive reflections of H-β zeolites, at 2θ values of 7.6°, 21.2° and 22.4°, were consistent with the typical diffraction lines of the referenced Beta zeolite, indicating a well-developed crystalline structure of Beta topology, formed by the intergrowth of polymorphs A and B [
29].
The crystallinity of the samples was defined on the basis of the three characteristics diffraction lines of Beta zeolite at Bragg angles of 7.5°, 21.3° and 22.4°, and it was calculated by using Equation (1) [
30], considering as reference the Beta zeolite sample with the highest intensity of the diffraction of these three lines.
Beta zeolites with a higher Si/Al ratio are characterized by a higher crystallinity (
Table 1), that is, in a good concordance with previous studies [
30,
31].
Sharper and more intense reflections of H-β6 (
Figure 1) indicate highly regular structures and slightly larger crystals that could be associated with the higher Al content (Si/Al = 6 for H-β6 versus Si/Al = 12 for H-β12). Moreover, all the reflection lines of H-β6 sample were shifted to lower 2θ values than for H-β12, indicating an increase in the unit cell size that could be associated to an increased concentration of longer Al-O bonds compared to Si-O bonds.
In contrast to other zeolites, the structure of the hydrated Beta zeolite shows a reversible change in the coordination of the framework Al atoms. Depending on the counter-ion it may balance the framework into a negative charge. Thus, it is generally accepted that in the hydrated protonic form of the Beta zeolite part of the framework Al atoms, with tetrahedral coordination in Na form, changes the coordination to octahedral [
32].
On the other hand, in an aggressive environment such as HNO
3 acid, a severe structural collapse of the Beta zeolite may occur. Under these conditions the produced mesoporosity is not useful because the resulted pores collapse and, therefore, the direct connection with the external surface of the zeolite particles is hindered. However, in the present case, the presence and the intensity of the typical diffraction lines of the Beta zeolites at 2θ of 7.6°, 21.2° and 22.5° (
Figure 2) certify that the dealumination conditions utilized in this work affected only in part the crystallinity of the parent zeolite, but no obvious broad peak due to the presence of an amorphous (silica-alumina) phase has been observed.
The X-ray diffraction (XRD) pattern of the DeAl-Beta sample prepared through the dealumination with oxalic acid (
Figure 2, red pattern) shows a change in the position of the narrow line from 2θ 22.48° to 22.75°, corresponding to a decrease of the d
302 spacing from 3.952 Å (H-β18) to 3.906 Å (DeAl-Beta). The dealumination with nitric acid changed the position of this diffraction line to 22.67° that correspond to a d
302 spacing of 3.917 Å. These shifts, in fact, confirm a larger contraction of the zeolite matrix [
33] by the treatment with oxalic acid. In conclusion, it emerges that the oxalic acid is more effective for the extraction of lattice aluminum than nitric acid. The extraction occurred without a collapse of the zeolite framework structure. This behavior should be associated with the dual nature of the oxalic acid. In relation to the framework aluminum it may act both as a hydrolyzing and a chelating agent. The dealumination process takes place via the formation of trioxalato aluminum complexes with a high complexation constant (logβ3 = 15.1) [
34].
Further, the treatment of the DeAl-beta samples with a niobium salt led to an increase in the d
302 spacing.
Figure 3 presents the XRD patterns of the Nb-treated samples, while the changes in the position of the diffraction line from 2θ 22.48° and the corresponding d
302 spacing are listed in
Table 2.
As
Table 2 shows, upon the incorporation of Nb into the DeAl-β samples, the increase of d
302 spacing compared to the DeAl-β reference depends on the dealumination route and loading of niobium. Finally, this corresponds to different expansions of the Nb-β zeolite matrix. However, the size of the niobium ions and the bond length of Nb-O (1.89 Å for tetracoordinated Nb (V) species in Beta zeolite) compared to that of Si-O (typically 1.60–1.65 Å in zeolites) [
35] do not support an insertion of Nb in the tetrahedral positions of the Beta matrix. The d
302 spacing values measured in this work are too small to sustain such an effect. Larger d
302 spacing values were measured for the samples dealuminated with oxalic acid (Nb(0.05)-β18O and Nb(0.05)-β37O,
Table 2, entries 8 and 10). In these cases the d
302 spacing values increased from 3.906 Å (DeAl-β18 (oxalic acid)) to 3.936 Å (Nb(0.05)-β18O) and from 3.912 Å (DeAl-β37 (oxalic acid)) to 3.969 Å (Nb(0.05)-β37O) and the shifts are close to those reported by Dzwigaj et al. [
36] (i.e., an increase of the d
302 spacing from 3.912 Å (SiBeta) to 3.954 Å (NbSiBeta, 2θ = 22.45°) for a 0.02 mol % Nb-SiBeta material prepared by the post synthesis methodology.
The inductively coupled plasma optical emission spectrometry (ICP-OES) measurements of the liquid phase separated after the Nb insertion step did not evidence the presence of niobium species suggesting, indeed, an entrapment of all niobia in the Beta zeolite structure. Therefore, it can be stated that for the samples dealuminated with nitric acid the low expansion of the Beta zeolite matrix is most probably the effect of its distortion by the non-framework pore encapsulation of Nb(V) nano-oxides. This process takes place without a real replacement of the tetrahedral aluminum in the zeolite framework. On the contrary, the dealumination with oxalic acid allows the insertion of Nb in the tetrahedral positions of the Beta matrix, at least partially.
The structural modifications of the zeolite matrix after the insertion of Nb were also evidenced by the FTIR study, confirming the XRD analysis results. In the 4000–2500 cm
−1 range, the FTIR spectra of all H-β and Nb(0.02)-β samples (
Figure 4 and
Figure 5) show a broad band assigned to the stretching vibrations of the -OH groups from H
2O adsorbed on the zeolite surface and/or existing in the zeolite channels (surface silanol groups, Si-OH). The sharp bands from this region can be associated with the asymmetric vibrations of some M-OH isolated groups. The FTIR spectra of H-β18 and H-β12 show typical -OH vibrations in Beta zeolites with bands of non-interacting bridging hydroxyls and the broad band ranging from 3620 to 3200 cm
−1 of mutually interacting Si(OH)Al groups. The OH stretching modes at wave numbers at 3642 cm
−1 (H-β12) and 3647 cm
−1 (H-β18) are due to the Si-O(H)-Al groups, at 3743 cm
−1 is assigned to terminal silanol (Si-OH) groups in small crystallites and high external surface area materials, while that at 3667 cm
−1 is attributed by some authors to AlO-H groups of perturbed and extra-framework Al atoms [
37].
The incorporation of Nb induced a reduction of all the above IR bands’ intensity and new bands centered at 3736 and 3671 cm
−1 were evidenced, most probably related to the presence of Nb(V)O-H and isolated SiO-H groups. At the same time, no absorption bands at 3555–3602 cm
−1 were evidenced, confirming the lack of Nb(IV), in accordance with a previous report of Tielens et al. [
36]. In 1500–500 cm
−1 range, corresponding to the framework vibration region, the presence of a new absorption band centered at 952 cm
−1 has also been evidenced (
Figure 6 and
Figure 7) for the Nb-based catalyst. According to literature, this band is generally taken as an indication of the metal incorporation into the zeolite framework and based on the reports of Corma et al. [
38] it can be attributed to the Si-O-Nb vibrations. However, it is more probable that the presence of such bonds correspond to the extra-framework isolated Nb(V)O-H species. The absence of a band located at 884 cm
−1 indicates the absence of the niobyl (-Nb=O bonds) species that may also account for the presence of the Nb
2O
5 clusters [
36].
A sharp peak around 1100 cm
−1 is usually noticed in zeolites and assigned to the asymmetric stretching of the SiO
4 tetrahedra. The shift to higher wave numbers is due to the presence of large amounts of other cations, since the Si-O bond distance is shorter than the Al-O one. Indeed, in the case of both the H-β rich-aluminum samples this characteristic band was evidenced at 1197 cm
−1 (H-β18) and 1202 cm
−1 (H-β12), respectively (
Figure 6 and
Figure 7). These bands can be associated with the remnant AlO-H group after the dealumination and Nb insertion, indicating either a partially replacement of the framework Al during the dealumination or the presence of the extra framework AlO
x(OH) species.
Indeed, it is already well known and documented that during the zeolite framework dealumination the Si-O-Al bonds are hydrolyzed, and tetrahedrally coordinated Al is extracted from the zeolite framework and deposited mostly as octahedrally coordinated mono- and even oligomeric Al-O extra-framework species [
39,
40]. Moreover, such residual extra-framework aluminum species adds Lewis acidity to the synthesized material [
41].
The geometry of Nb is dependent on the synthesis method [
36]. Indeed, the present procedure led to extra framework Nb(V)O-H species, while in zeolites prepared through a direct synthesis procedure tetrahedral species presenting a niobyl bond (-Nb=O) have been identified [
38]. However, in the case of the dealuminated samples with oxalic acid the presence of penta-coordinated framework Nb(V)O-H species should not be excluded.
2.2. Catalytic Behaviour
The aim of this study was to evaluate the catalytic activity of Nb-based zeolites samples for the selective CWO of glucose to SA. It is well known that the product distribution of WO remarkably depends on the experimental conditions and the reaction time as consequence of the instability of the primary products, their evolution over the time and the simultaneous concurrency of different reaction pathways. On the other hand, the catalytic performances are highly influenced by different catalytic system features as reaction conditions, catalytic active phase characteristics and the support nature. Therefore, it has been investigated the influence of the oxygen pressure, reaction temperature and reaction time upon the reaction products distribution.
Figure 8 presents the influence of the oxygen pressure and reaction time upon the distribution of the most important products, in the presence of the Nb(0.05)-β18 catalyst. As
Figure 8 shows, both the glucose conversion and selectivity to succinic acid increased with the oxygen pressure and reaction time. Practically, a total conversion of glucose was reached after 6 h, with a selectivity to succinic acid of 46.8%, at 18 bar O
2. However, the selectivity to succinic acid continued to increase in time and, after 12 h, reached 72.3%.
Lower loadings of niobium (i.e., 0.02 mol %) and reaction temperatures (i.e., 160 °C) led to lower selectivities to succinic acid (
Table 3). Interesting enough, the selectivity to succinic acid is favored by the presence of catalysts with higher Si/Al ratios. In accordance, the highest selectivity to SA (83.6%) for a total conversion of glucose was obtained in the presence of Nb(0.05)-β37.5, dealuminated with nitric acid (
Table 3). The corresponding Nb(0.05)-β37.5O sample, produced in the presence of oxalic acid, led to a selectivity of succinic acid of only 70% for a total conversion of glucose.
It is likely that lactic and glycolic acids are formed via the already proposed mechanism of the glucose degradation [
20]. On bifunctional catalysts, levulinic acid (LevA) can be also produced from glucose, usually in two steps involving the glucose isomerization to fructose on Lewis acid centers, followed by the fructose dehydration to LevA through 5-hydroxymethylfurfural (HMF) intermediate on Brønsted acid centers [
42,
43]. However, the dehydration of glucose to levulinic acid through a levoglucosane intermediate is also possible on materials presenting exclusively Brønsted acidity [
44]. In the absence of oxygen, at 180 °C and in the presence of Nb(0.05)-β18 catalyst, glucose is dehydrated to HMF (5-hydroxymethylfurfural) and levulinic acid with selectivities of 22.0% and 8.0%, respectively. However, comparing with the CWO reaction, in the presence of the same catalyst and under 18 atm O
2 (
Table 3, entry 3), the dehydration of glucose takes place with a much lower conversion (47.4%) and after 24 h. Unfortunately, during the glucose dehydration, besides HMF and levulinic acid, a large spectrum of other water soluble side products and unwanted HMF aldol-condensation byproducts, generically called “humins”, are obtained in high amounts. These humans block the active sites of the catalysts, leading to lower catalytic performances. The presence of molecular oxygen seems to be beneficial in avoiding the formation of such unwanted products (i.e., humins). In addition, for all CWO reactions the conversion of glucose is faster and orientated to the targeted reaction product, namely, succinic acid.
However, on Nb-zeolite catalysts the presence of levulinic acid suggests a different reaction pathway to SA compared to Ru-based catalysts [
13]. Using Ru-based catalysts only tartaric and fumaric acids have been detected in the reaction products. Under the WO conditions, hydroxy and hydroperoxy radicals are produced from the dissociation and oxidation of water (i.e., H
2O = •OH + •H; H
2O + O
2 = •OH + HO
2−). Hydrogen peroxide can also be formed from the hydroperoxy radicals recombination (i.e., 2HO
2− = H
2O
2 + O
2) [
45]. Then, these radicals together with the free oxygen can attack at the reducing end group, resulting in the opening of the glycosidic ring and the formation of carboxylic acids. In this case, a plausible pathway of the CWO of glucose to the SA product might involve a two-step mechanism: (A) a concerted heterogeneous-homogeneous free radical step leading to tartaric and oxalic acids, followed by the disproportionation of tartaric acid to fumaric and 2,3-dioxosuccinic acids; (B) the catalytic conversion of fumaric acid into SA.
Taking into consideration the literature reports and the experimental results obtained in this study, the formation of succinic acid can also be reasonably explained in the presence of the Nb-zeolite catalysts. According to Ziolek et al. [
46] the oxidation of cyclohexene, for instance, with hydrogen peroxide and in the presence of Nb-based catalysts, takes place through superoxo and peroxo species generated through the interaction of NbOH species with hydrogen peroxide:
Once formed, the hydroxyperoxy radicals may interact with hydrogen peroxide hydroxyl with the generation of superoxide radicals:
The hydroxyl radicals were identified as active species in the formation of cyclohexenediol [
47], while the concurrently produced superoxide radicals were considered to be trapped by the amorphous niobium oxide. The reaction equilibrium is in this way efficiently shifted towards the •OH radical formation:
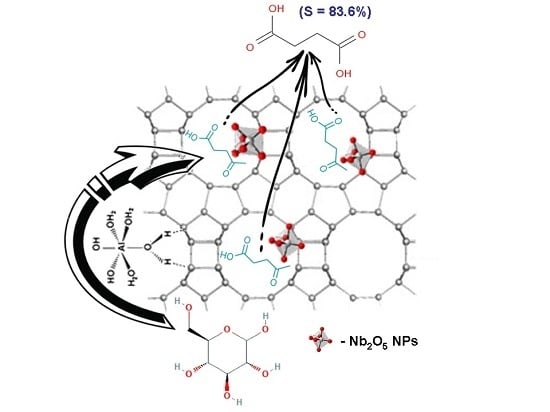
Similar species can also be formed under the CWO conditions used in this study. Therefore, a two-step mechanism for the production of succinic acid can be proposed, with the synergetic participation of two kinds of active sites: (i) the extra framework AlO
x(OH) species which dehydrate the glucose to levulinic acid, followed by; (ii) its subsequent oxidation, onto the nano-oxide Nb
2O
5 particles, located in the zeolite channels (as extra framework species and/or partly framework penta-coordinated species), in accordance to the above mechanisms (
Scheme 1).
These catalysts demonstrated stability under the harsh reaction conditions and can be recycled three times without noticeable variation of the glucose conversion and selectivity to succinic acid (
Table 4).
This remarkable stability in water can be due to the presence of the extra-framework aluminum species. When located on the external surface, they prevent the solubilization of the zeolite framework, as recently reported by Sels and co-workers [
48].