Adsorption of Cd (II) on Modified Granular Activated Carbons: Isotherm and Column Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. SEM Analysis
2.2. Physicochemical Characteristics of Activated Carbon
2.3. Cd (II) Adsorption Tests
2.3.1. Adsorption Isotherms
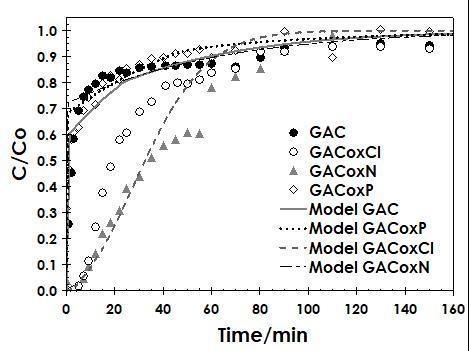
2.3.2. Fixed-Bed Column Studies
- A plug-flow pattern with axial dispersion (and negligible radial dispersion);
- Isothermal process due to the relatively high heat capacity of water;
- The adsorbent particles have an isotropic spherical shape;
- The accumulation of pollutant in the liquid contained in the pores of the adsorbent material is negligible
- A constant axial velocity.
3. Materials and Methods
3.1. Activated Carbon
3.2. Adsorbent Characterization
3.2.1. Textural Properties
3.2.2. SEM Analysis
3.2.3. pH at the Point of Zero Charge (pHPZC)
3.2.4. Boehm Titration Method
3.3. Cd (II) Adsorption Tests
3.3.1. Adsorption Isotherms
3.3.2. Fixed Bed Column Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.; Liu, L.; Wang, Y.; Luo, G.; Chen, X.; Yang, X.; Gao, B.; He, X. Integrated assessment of heavy metal contamination in sediments from a coastal industrial basin, NE China. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Madrid, L. “Heavy metals”: Reminding a long-standing and sometimes forgotten controversy. Geoderma 2012, 155, 128–129. [Google Scholar] [CrossRef]
- Alkaya, E.; Demirer, G. Greening of production in metal processing industry through process modifications and improved management practices. Resour. Conserv. Recycl. 2013, 77, 89–96. [Google Scholar] [CrossRef]
- Nordberg, G.; Nogawa, K.; Nordberg, M. Chapter 32—Cadmium. In Handbook on the Toxicology of Metals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 667–716. ISBN 978-0-444-59453-2. [Google Scholar]
- Avallone, B.; Cerciello, R.; Cretì, P.; Pizzoleo, C.; Scudiero, R.; Tizzano, M.; Panzuto, R.; Simoniello, P.; Montinari, M.; Motta, C. Long term exposure to cadmium: Pathological effects on kidney tubules cells in Sparus aurata juveniles. Aquat. Toxicol. 2017, 193, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, A.; Radcliffe, J.; Dietrich, K.N.; Jones, R.L.; Caldwell, K.; Rogan, W.J. Postnatal cadmium exposure, neurodevelopment, and blood pressure in children at 2, 5, and 7 years of age. Environ. Health Perspect. 2009, 117, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Barford, J.; Chen, G.; McKay, G. Kinetics and equilibrium studies for the removal of cadmium ions by ion exchange resin. J. Environ. Chem. Eng. 2014, 2, 698–707. [Google Scholar] [CrossRef]
- Angelis, C.; Galdiero, M.; Pivonello, C.; Salzano, C.; Gianfrilli, D.; Piscitelli, P.; Lenzi, A.; Colao, A.; Pivonello, R. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reprod. Toxicol. 2017, 73, 105–127. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Toxicological Profile for Cadmium, 2nd ed.; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012; pp. 75–99.
- Tinkov, A.; Filippini, T.; Ajsuvakova, P.; Aaseth, J.; Gluhcheva, Y.; Ivanova, J.; Bjørklund, G.; Skalnaya, M.; Gatiatulina, E.; Popova, E.; et al. The role of cadmium in obesity and diabetes. Sci. Total Environ. 2017, 601–602, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.; Edwards, J. Mechanisms of cadmium-induced proximal tubule injury: New insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Gao, Y.; Zhang, Y.; Wang, C.; Zhou, Y.; Hu, Y.; Shi, R.; Tian, Y. Effects of prenatal exposure to cadmium on neurodevelopment of infants in Shandong, China. Environ. Pollut. 2016, 211, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Matić, D.; Vlahović, M.; Kolarević, S.; Mataruga, V.; Ilijin, L.; Mrdaković, M.; Gačić, B. Genotoxic effects of cadmium and influence on fitness components of Lymantria dispar caterpillars. Environ. Pollut. 2016, 218, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, K.; Suwazono, Y. Itai-Itai Disease. In Encyclopedia of Environmental Health; Elsevier: Burlington, VT, USA, 2011; pp. 308–314. [Google Scholar]
- Zeng, F.; Wei, W.; Li, M.; Huang, R.; Yang, F.; Duan, Y. Heavy metal contamination in Rice-Producing soils of Hunan province, China and potential health risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Kobayashi, E.; Suwazono, Y.; Uetani, M.; Oishi, M.; Nakagawa, H.; Nogawa, K. Estimation of cumulative cadmium intake causing Itai-itai disease. Toxicol. Lett. 2005, 159, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Nnorom, I.C.; Osibanjo, O. Determination of metals in printed wiring boards of waste mobile phones. Toxicol. Environ. Chem. 2011, 93, 1557–1571. [Google Scholar] [CrossRef]
- McManus, M. Environmental consequences of the use of batteries in sustainable systems: Battery production. In Proceedings of the 2nd International Conference on Microgeneration and Related Technologies, Glasgow, UK, 4–6 April 2011; Available online: http://opus.bath.ac.uk/ 23361/1/p114v3.pdf (accessed on 24 January 2017).
- Balsamo, M.; Di Natale, F.; Erto, A.; Lancia, A.; Montagnaro, F.; Santoro, L. Gasification of coal combustion ash for its reuse as adsorbent. Fuel 2013, 106, 147–151. [Google Scholar] [CrossRef]
- Teeyakasem, W.; Nishijo, M.; Honda, R.; Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W. Monitoring of cadmium toxicity in a Thai population with high-level cadmium exposure. Toxicol. Lett. 2007, 169, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Chiu, K.; Lin, C.; Leu, H. Electrochemical treatment of wastewater: Selectivity of the heavy metals removal process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Hargreaves, A.; Vale, P.; Whelan, J.; Alibardi, L.; Constantino, C.; Dotro, G.; Cartmell, E.; Campo, P. Impacts of coagulation-flocculation treatment on the size distribution and bioavailability of trace metals (Cu, Pb, Ni, Zn) in municipal wastewater. Water Res. 2018, 128, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Folens, K.; Huysman, S.; Hulle, S.; Laing, G. Chemical and economic optimization of the coagulation-flocculation process for silver removal and recovery from industrial wastewater. Sep. Purif. Technol. 2017, 179, 145–151. [Google Scholar] [CrossRef]
- Morillo, D.; Faccini, M.; García, M. Highly efficient removal of heavy metal ions from polluted water using ion-selective polyacrylonitrile nanofibers. J. Environ. Chem. Eng. 2017. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Liu, S.; Zhang, J.; Yang, X. Molecular simulation of reverse osmosis for heavy metal ions using functionalized nanoporous graphenes. Comput. Mater. Sci. 2017, 139, 65–74. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, X.; Huang, W.; Lawless, D.; Feng, X. Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep. Purif. Technol. 2016, 158, 124–136. [Google Scholar] [CrossRef]
- Fang, L.; Li, L.; Qu, Z.; Xu, H.; Xu, J.; Yan, N. A novel method for the sequential removal and separation of multiple heavy metals from wastewater. J. Hazard. Mater. 2018, 342, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int. J. Environ. Sci. Technol. 2013, 10, 231–242. [Google Scholar] [CrossRef]
- Gong, J.; Liu, T.; Wang, X.; Hu, X.; Zhang, L. Efficient Removal of Heavy Metal Ions from Aqueous Systems with the Assembly of Anisotropic Layered Double Hydroxide Nanocrystals@Carbon Nanosphere. Environ. Sci. Technol. 2011, 45, 6181–6187. [Google Scholar] [CrossRef] [PubMed]
- Jeppu, G.P.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129–130, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part II. Models with more than two parameters. J. Hazard. Mater. 2007, 147, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Evaluation of Equilibrium Sorption Isotherm Equations. Open Chem. Eng. J. 2013, 7, 24–44. [Google Scholar] [CrossRef]
- Belhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Zhang, J.; Liang, S.; Chang, W.W.; Nguyen, D.D.; Liu, Y. Application of breakthrough biosorbent for removing heavy metals from synthetic and real wastewater in a lab-scale continuous fixed-bed column. Bioresour. Technol. 2017, 229, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Adsorptive removal of heavy metals from water using sodium titanate nanofibres loaded onto GAC in fixed-bed columns. J. Hazard. Mater. 2015, 287, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pérez, A.M.; Paradelo, M.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Heavy metal retention in copper mine soil treated with mussel shells: Batch and column experiments. J. Hazard. Mater. 2013, 248–249, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Borna, M.O.; Pirsaheb, M.; Niri, M.V.; Mashizie, R.K.; Kakavandi, B.; Zare, M.R.; Asadi, A. Batch and column studies for the adsorption of chromium(VI) on low-cost Hibiscus Cannabinus kenaf, a green adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 68, 80–89. [Google Scholar] [CrossRef]
- Ramrakhiani, L.; Ghosh, S.; Sarkar, S.; Majumdar, S. Heavy metal biosorption in multi component system on dried activated sludge: Investigation of adsorption mechanism by surface characterization. Mater. Today 2016, 3, 3538–3552. [Google Scholar] [CrossRef]
- Zhang, T.; Tu, Z.; Lu, G.; Duan, X.; Yi, X.; Guo, C.; Dang, Z. Removal of heavy metals from acid mine drainage using chicken eggshells in column mode. J. Environ. Manag. 2017, 188, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Achaw, O.W. A Study of the Porosity of Activated Carbons Using the Scanning Electron Microscope. In Scanning Electron Microscopy, 1st ed.; Kazmiruk, V., Ed.; InTech: Rijeka, Croatia, 2012; pp. 473–490. ISBN 978-953-51-0092-8. [Google Scholar]
- Kjellman, T.; Asahina, S.; Schmitt, J.; Impéror-Clerc, M.; Terasaki, O.; Alfredsson, M. Direct observation of plugs and intrawall pores in SBA-15 using low voltage high resolution scanning electron microscopy and the influence of solvent properties on plug-formation. Chem. Mater. 2013, 25, 4105–4112. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloid Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Sing, K.S.W. Assessment of Surface Area by Gas Adsorption. In Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Rouquerol, J., Rouquerol, F., Llewellyn, P., Maurin, G., Sing, K.S.W., Eds.; Elsevier Ltd.: Oxford, UK, 2014; pp. 237–263. ISBN 978-0-12-598920-6. [Google Scholar]
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbon (Origins). In Activated Carbon; Elsevier Science Ltd.: Oxford, UK, 2005; pp. 13–81. ISBN 978-0-08-044463-5. [Google Scholar]
- Wang, Y.; Shi, L.; Gao, L.; Wei, Q.; Cui, L.; Hu, L.; Yan, L.; Du, B. The removal of lead ions from aqueous solution by using magnetic hydroxypropyl chitosan/oxidized multiwalled carbon nanotubes composites. J. Colloid Interface Sci. 2015, 451, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Castro, A.; Mendonca, J. Characterization of acid functional groups of carbon dots by nonlinear regression data fitting of potentiometric titration curves. Appl. Surf. Sci. 2016, 370, 486–495. [Google Scholar] [CrossRef]
- Gao, Z.; Bandosz, T.J.; Zhao, Z.; Hand, M.; Qiua, J. Investigation of factors affecting adsorption of transition metals on oxidized carbon nanotubes. J. Hazard. Mater. 2009, 167, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Erto, A.; Giraldo, L.; Lancia, A.; Moreno-Piraján, J.C. A comparison between a low-cost sorbent and an activated carbon for the adsorption of heavy metals from water. Water Air Soil Pollut. 2013, 224, 1531–1541. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Abid, B.A.; Al-Najar, J.A. Removal of lead copper chromium and cobalt ions onto granular activated carbon in batch and fixed-bed adsorbers. Chem. Eng. J. 2009, 155, 647–653. [Google Scholar] [CrossRef]
- Vocciante, M.; Trofa, M.; D’Auria, T.; Giraldo, L.; Rodriguez-Estupiñán, P.; Moreno-Piraján, J.C.; Erto, A. A rigorous procedure for the design of adsorption units for the removal of cadmium and nickel from process wastewaters. J. Clean. Prod. 2014, 77, 35–46. [Google Scholar] [CrossRef]
- Erto, A.; Lancia, A.; Musmarra, D. Fixed-bed adsorption of trichloroethylene onto activated carbon. Chem. Eng. Trans. 2013, 32, 1969–1974. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Uguina, M.A.; Delgado, J.; Celemin, L.I. Adsorption of methyl ethyl ketone and trichloroethene from aqueous solutions onto activated carbon fixed-bed adsorbers. Sep. Purif. Technol. 2004, 37, 149–160. [Google Scholar] [CrossRef]
- Leyva-Ramos, R. Model simulation and analysis of surface diffusion of liquids in porous solids. Chem. Eng. Sci. 1985, 40, 799–807. [Google Scholar] [CrossRef]
- LeVan, M.D.; Carta, G. Adsorption and Ion Exchange. In Perry’s Chemical Engineers’ Handbook, 8th ed.; Perry, R.H., Green, D.W., Eds.; McGraw Hill: New York, NY, USA, 2008; pp. 1340–1405. ISBN 9780071422949. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |





| Sample | Slit Pore (%) | Cylindrical Pore (%) | Combined Pore (%) | |||
|---|---|---|---|---|---|---|
| NLDFT | QSDFT | NLDFT | QSDFT | NLDFT | QSDFT | |
| GAC | 2.34 | 1.27 | 1.97 | 1.46 | 1.46 | 1.03 |
| GACoxN | 2.22 | 1.61 | 2.47 | 1.61 | 0.970 | 0.678 |
| GACoxP | 2.69 | 1.15 | 3.42 | 2.22 | 1.31 | 1.22 |
| GACoxCl | 0.580 | 0.096 | 0.337 | 0.096 | 0.118 | 0.039 |
| Model | Sample | GAC | GACoxN | GACoxP | GACoxCl |
|---|---|---|---|---|---|
| Variable | |||||
| BET | SBET (m2·g−1) | 849 | 815 | 871 | 687 |
| C | 117 | 121 | 113 | 155 | |
| DA (P/P0 < 0.1) | Vmic (cm3·g−1) | 0.35 | 0.35 | 0.36 | 0.26 |
| Eo (kJ·mol−1) | 7.64 | 8.447 | 7.659 | 9.30 | |
| n | 1.80 | 1.40 | 1.80 | 2.00 | |
| Pore diameter (Å) | 14.2 | 13.4 | 14.2 | 13.4 | |
| QSDFT (P/P0 10−5 − 1) | VP (cm3·g−1) | 0.34 | 0.34 | 0.35 | 0.27 |
| Pore width (mode) (Å) | 7.85 | 7.53 | 7.85 | 7.85 |
| GAC | GACoxN | GACoxP | GACoxCl | |
|---|---|---|---|---|
| Carboxylic | 0.052 | 0.197 | 0.106 | 0.035 |
| Lactonic | 0.029 | 0.039 | 0.024 | 0.056 |
| Phenolic | 0.061 | 0.054 | 0.074 | 0.350 |
| Total Acidity | 0.142 | 0.290 | 0.204 | 0.441 |
| Total Basicity | 0.065 | 0.036 | 0.073 | 0.106 |
| Total Groups | 0.207 | 0.326 | 0.277 | 0.547 |
| pHPZC | 5.4 | 3.4 | 6.2 | 7.2 |
| Samples | Langmuir Model | Sips Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Q0 (mg/L) | KL (L/mg) | R2 | RL (50–500 mg·L−1) | QS (mg/L) | KS (L/mg) | ns | R2 | |
| GAC | 93.3 | 0.0012 | 0.991 | 0.67–0.17 | 185 | 0.0044 | 0.519 | 0.993 |
| GACoxN | 41.6 | 0.0051 | 0.999 | 0.80–0.29 | 51.5 | 0.0063 | 0.887 | 0.989 |
| GACoxP | 131 | 0.0014 | 0.993 | 0.95–0.67 | 71.1 | 0.0022 | 1.067 | 0.996 |
| GACoxCl | 40.6 | 0.0203 | 0.993 | 0.57–0.12 | 190 | 0.0275 | 0.385 | 0.970 |
| Adsorbent | MTCext (s−1) | (s−1) | Ds (m2·s−1) | MTCtot (s−1) |
|---|---|---|---|---|
| GAC | 5.69 × 10−1 | 1.35 × 10−3 | 4.6 × 10−11 | 2.17 × 10−1 |
| GACoxP | 5.69 × 10−1 | 7.72 × 10−3 | 3.2 × 10−11 | 2.14 × 10−1 |
| GACoxN | 5.69 × 10−1 | 2.43 × 10−3 | 1.0 × 10−11 | 1.01 × 10−1 |
| GACoxCl | 5.69 × 10−1 | 9.76 × 10−3 | 4.2 × 10−11 | 3.86 × 10−1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Estupiñán, P.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of Cd (II) on Modified Granular Activated Carbons: Isotherm and Column Study. Molecules 2017, 22, 2280. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122280
Rodríguez-Estupiñán P, Erto A, Giraldo L, Moreno-Piraján JC. Adsorption of Cd (II) on Modified Granular Activated Carbons: Isotherm and Column Study. Molecules. 2017; 22(12):2280. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122280
Chicago/Turabian StyleRodríguez-Estupiñán, Paola, Alessandro Erto, Liliana Giraldo, and Juan Carlos Moreno-Piraján. 2017. "Adsorption of Cd (II) on Modified Granular Activated Carbons: Isotherm and Column Study" Molecules 22, no. 12: 2280. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122280