Electrospun Fibers of Cyclodextrins and Poly(cyclodextrins)
Abstract
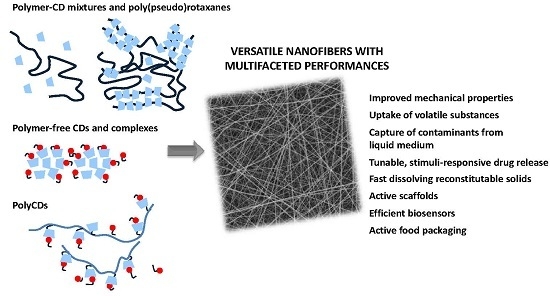
:1. Introduction
2. Electrospun Mats of Mixtures of Cyclodextrins and Polymers
2.1. Electrospining Methods for Cyclodextrin Incorporation
2.1.1. Uniaxial Processing
Blends of Cyclodextrins and Polymers
Polypseudorotaxanes
Post-spinning Modifications
2.1.2. Coaxial processing
3. Electrospun Mats of Polymer-free Cyclodextrins
4. Electrospun Mats of Cyclodextrin-based Polymers
5. Conclusions and Future Outlook
Acknowledgments
Conflicts of Interest
Abbreviations
| AHL | N-acyl-L-homoserine lactone |
| CD | cyclodextrin |
| CFM | chloroform |
| CNTs | multiwalled carbon nanotubes |
| DMAc | dimethylacetamide |
| DMF | dimethylformamide |
| DMSO | dimethylsulfoxide |
| ECM | extracellular matrix |
| HCPT | hydroxycamptothecin |
| HMDSO | hexamethyldisiloxane |
| HFIP | 1,1,1,3,3,3-hexafluoro-2-propanol |
| HPC | hydroxypropyl cellulose |
| HPβCD | hydroxypropyl-β-cyclodextrin |
| HPγCD | hydroxypropyl-γ-cyclodextrin |
| MβCD | methyl-β-cyclodextrin |
| PAA | poly(acrylic acid) |
| PBS | phosphate buffer saline |
| PCL | poly(ε-caprolactone) |
| PDADMAC | poly(diallyldimethylammonium chloride) |
| PEG | polyethylene glycol |
| PELA | poly(DL-lactic acid)-poly(ethylene glycol) |
| PEO | poly(ethylene oxide) |
| PLA | polylactic acid |
| PMAA | poly(methacrylic acid) |
| PMMA | poly(methyl methacrylate) |
| POSS-NH2 | amino polyhedral oligomeric silsesquioxanes |
| PVA | polyvinyl alcohol |
| PVdF | polyvinylidene fluoride |
| PVP | polyvinyl pyrrolidone |
| SBEβCD | sulfobutylether-β-cyclodextrin |
References
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Supaphol, P.; Suwantong, O.; Sangsanoh, P.; Sowmya, S.; Jayakumar, R.; Nair, S.V. Electrospinning of biocompatible polymers and their potentials in biomedical applications. Adv. Polym. Sci. 2012, 246, 213–240. [Google Scholar]
- Kriegel, C.; Arrechi, A.; Kit, K.; McClements, D.J.; Weiss, J. Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit. Rev. Food Sci. Nutr. 2008, 48, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical attributes of nanofibers: Preparation, drug loading, and tissue regeneration. Int. J. Pharm. 2015, 484, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Meinel, A.J.; Germershaus, O.; Luhmann, T.; Merkle, H.P.; Meinel, L. Electrospun matrices for localized drug delivery: Current technologies and selected biomedical applications. Eur. J. Pharm. Biopharm. 2012, 81, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.F.; Shakir, I.; Hussain, R. Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J. Mater. Sci. 2013, 48, 3027–3054. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Zarghami, N.; Melat Yar, H.; Akbarzadeh, A. Nanofiber: Synthesis and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2014, 44, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gómez, L.; Montini Ballarin, F.; Abraham, G.A.; Concheiro, A.; Alvarez-Lorenzo, C. Random and aligned PLLA: PRGF electrospun scaffolds for regenerative medicine. J. Appl. Polym. Sci. 2015, 132, 41372–41381. [Google Scholar] [CrossRef]
- Díaz-Gómez, L.; Alvarez-Lorenzo, C.; Concheiro, A.; Silva, M.; Dominguez, F.; Sheikh, F.A.; Cantu, T.; Desai, R.; Garcia, V.L.; Macossay, J. Biodegradable electrospun nanofibers coated with platelet-rich plasma for cell adhesion and proliferation. Mater. Sci. Eng. C 2014, 40, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Szabó, P.; Kállai-Szabó, B.; Zelkó, R. Incorporating small molecules or biologics into nanofibers for optimized drug release: A review. Int. J. Pharm. 2015, 494, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Ramakirshna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Ejaz Ahmed, F.; Singh Lalia, B.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Kaur, S.; Sundarrajan, S.; Rana, D.; Sridhar, R.; Gopal, R.; Matsuura, T.; Ramakrishna, S. Review: The characterization of electrospun nanofibrous liquid filtration membranes. J. Mater. Sci. 2014, 49, 6143–6159. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C. Effects of working parameters on electrospinning. In One-Dimensional Nanostructures. Electrospinning Technique and Unique Nanofibers; Springer: Heidelberg, Germany, 2013; pp. 15–28. [Google Scholar]
- Moghe, A.K.; Gupta, B.S. Co-axial electrospinning for nanofiber structures: Preparation and applications. Polym. Rev. 2008, 48, 353–377. [Google Scholar] [CrossRef]
- Yu, J.H.; Fridrikh, S.V.; Rutledge, G.C. The role of elasticity in the formation of electrospun fibers. Polymer 2006, 47, 4789–4797. [Google Scholar] [CrossRef]
- Anu Bushani, J.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Sell, S.A.; McClure, M.J.; Garg, K.; Wolfe, P.S.; Bowlin, G.L. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Pose-Vilarnovo, B.; Rodriguez-Tenreiro, C.; dos Santos, J.F.R.; Vazquez-Doval, J.; Concheiro, A.; Alvarez-Lorenzo, C.; Torres-Labandeira, J.J. Modulating drug release with cyclodextrins in hydroxypropyl methylcellulose gels and tablets. J. Control. Release 2004, 94, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and functionalization of polymers with cyclodextrins: Current applications and future prospects. Molecules 2014, 19, 14066–14079. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Uyar, T. Encapsulation of vanillin/cyclodextrin inclusion complex in electrospun polyvinyl alcohol (PVA) nanowebs: Prolonged shelf-life and high temperature stability of vanillin. Food Chem. 2012, 133, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Celebioglu, A.; Sen, H.S.; Durgun, E.; Uyar, T. Molecular entrapment of volatile organic compounds (VOCs) by electrospun cyclodextrin nanofibers. Chemosphere 2016, 144, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Borbás, E.; Balogh, A.; Bocz, K.; Müller, J.; Kiserdei, E.; Vigh, T.; Sinkó, B.; Marosi, A.; Halász, A.; Dohányos, Z.; et al. In vitro dissolution-permeation evaluation of an electrospun cyclodextrin-based formulation of aripiprazole using µFlux™. Int. J. Pharm. 2015, 491, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Burns, N.A.; Burroughs, M.C.; Gracz, H.; Pritchard, C.Q.; Brozena, A.H.; Willoughby, J.; Khan, S.A. Cyclodextrin facilitated electrospun chitosan nanofibers. RSC Adv. 2015, 5, 7131–7137. [Google Scholar] [CrossRef]
- Li, L.; Hsieh, Y.L. Ultra-fine polyelectrolyte fibers from electrospinning of poly(acrylic acid). Polymer 2005, 46, 5133–5139. [Google Scholar] [CrossRef]
- Boas, M.; Gradys, A.; Vasilyev, G.; Burman, M.; Zussman, E. Electrospinning polyelectrolyte complexes: pH-responsive fibers. Soft Matter 2015, 11, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.F.; Gong, R.H.; Rigout, M. Optimization of the process variables for electrospinning of poly(ethylene oxide)-loaded hydroxypropyl-β-cyclodextrin nanofibres. J. Text. I. 2015, 107, 1–11. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, L.; Liang, Q.; Zhang, X.; Guan, H.; Xu, X.; Chen, X.; Jing, X. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J. Control. Release 2005, 105, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Canbolat, M.F.; Celebioglu, A.; Uyar, T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surf. B 2014, 115, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Z.; Williams, G.R.; Hou, X.X.; Zhu, L.-M. Electrospun curcumin-loaded fibers with potential biomedical applications. Carbohydr. Polym. 2013, 94, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Szentivanyi, A.; Chakradeo, T.; Zernetsch, H.; Glasmacher, B. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Adv. Drug Deliv. Rev. 2011, 63, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C 2016, 63, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast-acting clotrimazole composited PVP/HPβCD nanofibers for oral candidiasis application. Pharm. Res. 2014, 31, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fabrication and evaluation of nanostructured herbal oil/hydroxypropyl-β-cyclodextrin/polyvinylpyrrolidone mats for denture stomatitis prevention and treatment. AAPS PharmSciTech 2016, 17, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Vigh, T.; Horvathova, T.; Balogh, A.; Soti, P.L.; Dravavolgyi, G.; Nagy, Z.K.; Marosi, G. Polymer-free and polyvinylpirrolidone-based electrospun solid dosage forms for drug dissolution enhancement. Eur. J. Pharm. Sci. 2013, 49, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Azarbayjani, A.; Chan, S.Y. Single and Multi-Layered Nanofibers for Rapid and Controlled Drug Delivery. Chem. Pharm. Bull. 2010, 58, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Effects on drug permeation through biological membranes. J. Pharm. Pharmacol. 2011, 63, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Simoes, S.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular cyclodextrin-based drug nanocarriers. Chem. Commun. 2015, 51, 6275–6289. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Dogan, S.M.; Tekinay, T.; Uyar, T. Release and antibacterial activity of allylisothiocyanate/β-cyclodextrin complex encapsulated in electrospun nanofibers. Colloids Surf. B 2014, 120, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Uyar, T.; Hacaloglu, J.; Besenbacher, F. Electrospun polystyrene fibers containing high temperature stable volatile fragrance/flavor facilitated by cyclodextrin inclusion complexes. React. Funct. Polym. 2009, 69, 145–150. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.H.; Feng, K.; Liu, F.J.; Lou, W.Y.; Li, N.; Zong, M.H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Uyar, T. Antioxidant activity and photostability of α-tocopherol/β-cyclodextrin inclusion complex encapsulated electrospun polycaprolactone nanofibers. Eur. Polym. J. 2016, 79, 140–149. [Google Scholar] [CrossRef]
- Lemma, S.M.; Scampicchio, M.; Mahon, P.J.; Sbarski, I.; Wang, J.; Kingshott, P. Controlled release of retinyl acetate from β-cyclodextrin functionalized poly(vinyl alcohol) electrospun nanofibers. J. Agric. Food Chem. 2015, 63, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Azarbayjani, A.; Qun, L.; Chan, Y.W.; Chan, S.Y. Novel vitamin and gold-loaded nanofiber facial mask for topical delivery. AAPS PharmSciTech 2010, 11, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Yao, J.; Shao, Z.; Chen, X. Tamoxifen-loaded silk fibroin electrospun fibers. Mater. Lett. 2016, 178, 31–34. [Google Scholar] [CrossRef]
- Aytac, Z.; Sen, H.S.; Durgun, E.; Uyar, T. Sulfisoxazole/cyclodextrin inclusion complex incorporated in electrospun hydroxypropyl cellulose nanofibers as drug delivery system. Colloids Surf. B 2015, 128, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Canbolat, M.F.; Savas, H.B.; Gultekin, F. Improved catalytic activity by catalase immobilization using γ-cyclodextrin and electrospun PCL nanofibers. J. Appl. Polym. Sci. 2017, 134, 44404. [Google Scholar] [CrossRef]
- Luo, X.; Xie, C.; Wang, H.; Liu, C.; Yan, S.; Li, X. Antitumor activities of emulsion electrospun fibers with core loading of hydroxycamptothecin via intratumoral implantation. Int. J. Pharm. 2012, 425, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous materials and their applications. Annu. Rev. Mater. Res. 2006, 36, 333–368. [Google Scholar] [CrossRef]
- Kim, T.G.; Ragupathy, D.; Gopalan, A.I.; Lee, K.P. Electrospun carbon nanotubes-gold nanoparticles embedded nanowebs: Prosperous multi-functional nanomaterials. Nanotechnol. 2010, 21, 134021. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Aytac, Z.; Umu, O.C.O.; Dana, A.; Tekinay, T.; Uyar, T. One-step synthesis of size-tunable Ag nanoparticles incorporated in electrospun PVA/cyclodextrin nanofibers. Carbohydr. Polym. 2014, 99, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, J.; Li, C.; Zhang, J. Functionalization of electrospun β-cyclodextrin/polyacrylonitrile (PAN) with silver nanoparticles: Broad-spectrum antibacterial property. Appl. Surf. Sci. 2012, 261, 499–503. [Google Scholar] [CrossRef]
- Netsuwan, P.; Mimiya, H.; Baba, A.; Sriwichai, S.; Shinbo, K.; Kato, K.; Kaneko, F.; Phanichphant, S. Long-range surface plasmon resonance immunosensor based on water-stable electrospun poly(acrylic acid) fibers. Sens. Actuators B Chem. 2014, 204, 770–776. [Google Scholar] [CrossRef]
- Barhate, R.S.; Ramakrishna, S. Nanofibrous filtering media: Filtration problems and solutions from tiny materials. J. Membr. Sci. 2007, 296, 1–8. [Google Scholar] [CrossRef]
- Uyar, T.; Havelund, R.; Nur, Y.; Balan, A.; Hacaloglu, J.; Toppare, L.; Besenbacher, F.; Kingshott, P. Cyclodextrin functionalized poly(methyl methacrylate) (PMMA) electrospun nanofibers for organic vapors waste treatment. J. Membr. Sci. 2010, 365, 409–417. [Google Scholar] [CrossRef]
- Kaur, S.; Kotaki, M.; Ma, Z.; Gopal, R.; Ramakrishna, S. Oligosaccharide functionalized nanofibrous membrane. Int. J. Nanosci. 2006, 5, 1–11. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Bai, J.; Xu, T.; Sun, W.; Xu, W.; Huang, Y. Synthesis of α-, β- and γ-cyclodextrin/poly(acrylonitrile) composite nanofibers and their applications to Cu(II) ion adsorption. Polym. Plast. Technol. Eng. 2014, 53, 513–519. [Google Scholar] [CrossRef]
- Kayaci, F.; Sen, H.S.; Durgun, E.; Uyar, T. Electrospun nylon 6,6 nanofibers functionalized with cyclodextrins for removal of toluene vapor. J. Appl. Polym. Sci. 2015, 132, 41941. [Google Scholar] [CrossRef]
- Meng, Q.; Bai, J.; Li, C.; Huang, Y.; Liu, H.; Li, H. Electrospun functional cyclodextrins/polystyrene (PS) composite nanofibers and their applications for sorption of Cu (II) ions under aqueous solution. Nanosci. Nanotechnol. Lett. 2014, 6, 289–294. [Google Scholar] [CrossRef]
- Harada, A. Preparation and structures of supramolecules between cyclodextrins and polymers. Coord. Chem. Rev. 1996, 148, 115–133. [Google Scholar] [CrossRef]
- Higashi, T.; Motoyama, K.; Arima, H. Cyclodextrin-based polyrotaxanes and polypseudorotaxanes as drug delivery carriers. J. Drug Deliv. Sci. Technol. 2013, 23, 523–529. [Google Scholar] [CrossRef]
- Tan, S.; Ladewig, K.; Fu, Q.; Blencowe, A.; Qiao, G.G. Cyclodextrin-based supramolecular assemblies and hydrogels: Recent advances and future perspective. Macromol. Rapid Commun. 2014, 35, 1166. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Chung, C.C.; Aguda, R.; Boy, R.; Hartman, M.; Mehraban, N.; Gupta, B.S.; Tonelli, A.E. Correlation of the stoichiometries of poly(ε-caprolactone) and α-cyclodextrin pseudorotaxanes with their solution rheology and the molecular orientation, crystallite size, and thermomechanical properties of their nanofibers. RSC Adv. 2016, 6, 111326–111336. [Google Scholar] [CrossRef]
- Harada, A.; Kamachi, M. Complex formation between poly(ethylene glycol) and α-cyclodextrin. Macromolecules 1990, 23, 2821–2823. [Google Scholar] [CrossRef]
- Simões, S.M.N.; Veiga, F.; Torres-Labandeira, J.J.; Ribeiro, A.C.F.; Concheiro, A.; Alvarez-Lorenzo, C. Syringeable self-assembled cyclodextrin gels for drug delivery. Curr. Top. Med. Chem. 2014, 14, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Uyar, T.; Kingshott, P.; Besenbacher, F. Electrospinning of cyclodextrin-pseudopolyrotaxane nanofibers. Angew. Chem. Int. Ed. 2008, 47, 9108–9111. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Gupta, B.S.; Tonelli, A.E. Estimation of the poly (ε-caprolactone) [PCL] and α-cyclodextrin [α-CD] stoichiometric ratios in their inclusion complexes [ICs], and evaluation of porosity and fiber alignment in PCL nanofibers containing these ICs. Data Brief 2015, 5, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Gupta, B.S.; Tonelli, A.E. Poly(ε-caprolactone) nanowebs functionalized with 〉- and γ-cyclodextrins. Biomacromolecules 2014, 15, 4122–4133. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Gupta, B.S.; Tonelli, A.E. Enhanced mechanical properties of poly (ε-caprolactone) nanofibers produced by the addition of nonstoichiometric inclusion complexes of poly (ε-caprolactone) and α-cyclodextrin. Polymer 2015, 76, 321–330. [Google Scholar] [CrossRef]
- Narayanan, G.; Aguda, R.; Hartman, M.; Chung, C.C.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Fabrication and characterization of poly(ε-caprolactone)/α-cyclodextrin pseudorotaxane nanofibers. Biomacromolecules 2016, 17, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Ormond, B.R.; Gupta, B.S.; Tonelli, A.E. Efficient wound odor removal by β-cyclodextrin functionalized poly (ε-caprolactone) nanofibers. J. Appl. Polym. Sci. 2015, 132, 42782. [Google Scholar] [CrossRef]
- Zhan, J.; Singh, A.; Zhang, Z.; Huang, L.; Elisseeff, J.H. Multifunctional aliphatic polyester nanofibers for tissue engineering. Biomatter 2012, 2, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin-epichlorohydrin polymers. Progr. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Concheiro, A.; Alvarez-Lorenzo, C. Chemically cross-linked and grafted cyclodextrin hydrogels: From nanostructures to drug-eluting medical devices. Adv. Drug Deliv. Rev. 2013, 65, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Jiang, Z.; Wang, C. Water-insoluble sericin/β-cyclodextrin/PVA composite electrospun nanofibers as effective adsorbents towards methylene blue. Colloid Surf. B 2015, 136, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Wen, Q.; Liu, Q.; Yang, Q.; Li, Y. Electrospinning preparation of β-cyclodextrin/glutaraldehyde crosslinked PVP nanofibrous membranes to adsorb dye in aqueous solution. Chem. Res. Chin. Univ. 2014, 30, 1057–1062. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Zhang, Z.; Wang, L.; Chen, Z.; Xiang, S. The cooperative utilization of imprinting, electro-spinning and a pore-forming agent to synthesise β-cyclodextrin polymers with enhanced recognition of naringin. RSC Adv. 2013, 3, 25396–25402. [Google Scholar] [CrossRef]
- Nasuno, E.; Umemura, T.; Ogi, T.; Okano, C.; Kawanago, T.; Iimura, K.; Morohoshi, T.; Ikeda, T.; Kato, N. Inhibitory effects of quorum sensing in Serratia marcescens AS-1 by electrospun polyvinyl alcohol fibers immobilized with cyclodextrin. Trans. Mater. Res. Soc. Jpn. 2012, 37, 593–596. [Google Scholar] [CrossRef]
- Brackman, G.; Garcia-Fernandez, M.J.; Lenoir, J.; de Meyer, L.; Remon, J.P.; De Beer, T.; Concheiro, A.; Alvarez-Lorenzo, C.; Coenye, T. Dressings loaded with cyclodextrin-hamamelitannin complexes increase staphylococcus aureus susceptibility towards antibiotics both in single as well as in mixed biofilm communities. Macromol. Biosci. 2016, 16, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kong, J.; Yao, X.; Zhao, C.; Dong, Y.; Lu, X. Polydopamine-assisted attachment of β-cyclodextrin on porous electrospun fibers for water purification under highly basic condition. Chem. Eng. J. 2015, 270, 101–109. [Google Scholar] [CrossRef]
- Yuan, G.; Prabakaran, M.; Qilong, S.; Lee, J.S.; Chung, I.M.; Gopiraman, M.; Song, K.H.; Kim, I.S. Cyclodextrin functionalized cellulose nanofiber composites for the faster adsorption of toluene from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 70, 352–358. [Google Scholar] [CrossRef]
- Forouharshad, M.; Putti, M.; Basso, A.; Prato, M.; Monticelli, O. Biobased system composed of electrospun sc-PLA/POSS/Cyclodextrin fibers to remove water pollutants. ACS Sustain. Chem. Eng. 2015, 3, 2917–2924. [Google Scholar] [CrossRef]
- Celebioglu, A.; Demirci, S.; Uyar, T. Cyclodextrin-grafted electrospun cellulose acetate nanofibers via “click” reaction for removal of phenanthrene. Appl. Surf. Sci. 2014, 305, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Kayaci, F.; Aytac, Z.; Uyar, T. Surface modification of electrospun polyester nanofibers with cyclodextrin polymer for the removal of phenanthrene from aqueous solution. J. Hazard. Mater. 2013, 261, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Noreña-Caro, D.; Alvarez-Lainez, M. Functionalization of polyacrylonitrile nanofibers with β-cyclodextrin for the capture of formaldehyde. Mater. Des. 2016, 95, 632–640. [Google Scholar] [CrossRef]
- Vysloužilová, L.; Buzgo, M.; Pokorný, P.; Chvojka, J.; Míčková, A.; Rampichová, M.; Kula, J.; Pejchar, K.; Bílek, M.; Lukáš, D.; et al. Needleless coaxial electrospinning. Int. J. Pharm. 2017, 1–2, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.; Hebraud, A.; Gallet, S.; Lapp, A.; Pollet, E.; Averous, L.; Schlatter, G. Star-pseudopolyrotaxane organized in nanoplatelets for poly(ε-caprolactone)-based nanofibrous scaffolds with enhanced surface reactivity. Macromol. Rapid Commun. 2015, 36, 292–297. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.G.; Layman, J.M.; Cashion, M.P.; Long, T.E. Phospholipid nonwoven electrospun membranes. Science 2006, 311, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Cashion, M.P.; Li, X.; Geng, Y.; Hunley, M.T.; Long, T.E. Gemini surfactant electrospun membranes. Langmuir 2010, 26, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Manasco, J.L.; Saquing, C.D.; Tang, C.; Khan, S.A. Cyclodextrin fibers via polymer-free electrospinning. RSC Adv. 2012, 2, 3778–3784. [Google Scholar] [CrossRef]
- Fulop, Z.; Kurkov, S.V.; Nielsen, T.T.; Larsen, K.L.; Loftsson, T. Self-assembly of cyclodextrins: Formation of cyclodextrin polymer based nanoparticles. J. Drug Deliv. Sci. Technol. 2012, 22, 215–221. [Google Scholar] [CrossRef]
- Messner, M.; Kurkov, S.V.; Jansook, P.; Loftsson, T. Self-assembled cyclodextrin aggregates and nanoparticles. Int. J. Pharm. 2010, 387, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ryzhakov, A.; Thi, T.D.; Stappaerts, J.; Bertoletti, L.; Kimpe, K.; Rodrigues Sá Couto, A.; Saokham, P.; Van den Mooter, G.; Augustijns, P.; Somsen, G.W.; et al. Self-assembly of cyclodextrins and their complexes in aqueous solutions. J. Pharm. Sci. 2016, 105, 2556–2569. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Electrospun nanofibers from native cyclodextrins. J. Colloid Interface Sci. 2013, 404, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Kang, Y.; Ku, M.; Yang, Y.-H.; Jung, S.; Kim, H. Preparation of β-cyclodextrin fiber using electrospinning. RSC Adv. 2013, 3, 14983–14987. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun gamma-cyclodextrin (γ-CD) nanofibers for the entrapment of volatile organic compounds. RSC Adv. 2013, 3, 22891–22895. [Google Scholar] [CrossRef]
- Kida, T.; Sato, S.I.; Yoshida, H.; Teragaki, A.; Akashi, M. 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) as a novel and effective solvent to facilely prepare cyclodextrin-assembled materials. Chem. Commun. 2014, 50, 14245–14248. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Polymer-free nanofibers from cyclodextrin derivates. Nanoscale 2012, 4, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, M.; Zha, B.; Diao, G. Correlation of polymer-like solution behaviors with electrospun fiber formation of hydroxypropyl-β-cyclodextrin and the adsorption study on the fiber. Phys. Chem. Chem. Phys. 2012, 14, 9729–9737. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Keskin, N.O.S.; Tekinay, T.; Uyar, T. Electrospinning of polymer-free cyclodextrin/geraniol-inclusion complex nanofibers: enhanced shelf-life of geraniol with antibacterial and antioxidant properties. RSC Adv. 2016, 6, 46089–46099. [Google Scholar] [CrossRef]
- Celebioglu, A.; Umu, O.C.O.; Tekinay, T.; Uyar, T. Antibacterial electrospun nanofibers from triclosan/cyclodextrin inclusion complexes. Colloid Surf. B 2014, 116, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Kayaci-Senirmak, F.; Kusku, S.I.; Durgun, E.; Uyar, T. Polymer-free nanofibers from vanillin/cyclodextrin inclusion complexes: High thermal stability, enhanced solubility and antioxidant property. Food Funct. 2016, 7, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Nielsen, S.R.; Uyar, T.; Zhang, S.; Zafar, A.; Dong, M.; Besenbacher, F. Electrospun UV-responsive supramolecular nanofibers from a cyclodextrin–azobenzene inclusion complex. J. Mater. Chem. C 2013, 1, 850–855. [Google Scholar] [CrossRef]
- Balogh, A.; Horvathova, T.; Fulop, Z.; Loftsson, T.; Harasztos, A.H.; Marosi, A.; Nagy, Z.K. Electroblowing and electrospinning of fibrous diclofenac sodium cyclodextrin complex-based reconstitution injection. J. Drug Deliv. Sci. Technol. 2015, 26, 28–34. [Google Scholar] [CrossRef]
- González-Gaitano, G.; Rodríguez, P.; Isasi, J.R.; Fuentes, M.; Tardajos, G.; Sánchez, M. The aggregation of cyclodextrins as studied by photon correlation spectroscopy. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 101–105. [Google Scholar] [CrossRef]
- Sousa, F.B.; Guerreiro, J.D.T.; Ma, M.; Anderson, D.G.; Drum, C.L.; Sinisterra, R.D.; Langer, R. Photo-response behavior of electrospun nanofibers based on spiropyran-cyclodextrin modified polymer. J. Mater. Chem. 2010, 20, 9910–9917. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Suarez, D.; Barbosa Rocha, J.C.; Novaes de Carvalho Teixeira, A.V.; Cortés, M.E.; de Sousa, F.B.; Sinisterra, R.D. Electrospun nanofibers of polyCD/PMAA polymers and their potential application as drug delivery system. Mater. Sci. Eng. C 2015, 54, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Costoya, A.; Montini Ballarin, F.; Llovo, J.; Concheiro, A.; Abraham, G.A.; Alvarez-Lorenzo, C. HMDSO-plasma coated electrospun fibers of poly(cyclodextrin)s for antifungal dressings. Int. J. Pharm. 2016, 513, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, S.; Degoutin, S.; Tabary, N.; Cazaux, F.; Maton, M.; Gaucher, V.; Janus, L.; Neut, C.; Chai, F.; Blanchemain, N.; et al. Triclosan loaded electrospun nanofibers based on a cyclodextrin polymer and chitosan polyelectrolyte complex. Int. J. Pharm. 2016, 513, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Rodell, C.B.; Kim, I.L.; Wade, R.J.; Burdick, J.A. Ordered, adherent layers of nanofibers enabled by supramolecular interactions. J. Mater. Chem. B 2014, 2, 8110–8115. [Google Scholar] [CrossRef] [PubMed]







| CD or IC | Concentration (% w/v) | Solvents (v/v) | Application | Reference |
|---|---|---|---|---|
| αCD | 160 | 10% NaOH | [110] | |
| βCD | 150 | |||
| βCD | 60 | DMF/1-ethyl-3-methylimidazolium acetate (3:7 or 4:6) | [111] | |
| γCD | 140 | DMSO/Water (50:50) | Entrapment volatile organic compounds (aniline, toluene) | [112] |
| αCD | 12.5 | 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) | [113] | |
| βCD | 12.5 | |||
| γCD | 7.5 | |||
| MβCD | 140; 160 | Water | [114] | |
| 140; 160 | DMF | |||
| 160 | DMAc | |||
| HPβCD | 160 | Water | ||
| 120 | DMF | |||
| 120 | DMAc | |||
| HPγCD | 160 | Water | ||
| 125 | DMF | |||
| 125 | DMAc | |||
| HPβCD | 160 | Water | Entrapment of volatile organic compounds (aniline, benzene) | [34] |
| 120 | DMF | |||
| HPγCD | 160 | Water | ||
| 125 | DMF | |||
| HPβCD | 61.4 | DMF | [115] | |
| (HPβCD; HPγCD; MβCD)-Geraniol-IC | 200 | Water | Prolonged releasing systems with antioxidant and antibacterial activity | [116] |
| (HPβCD; HPγCD)-Triclosan-IC | 160 | Water | Wound dressings with antibacterial activity | [117] |
| HPβCD-Vanillin-IC | 160 | Water | Food or pharmaceutical products | [118] |
| 120 | DMF, DMAc | |||
| HPγCD-Vanillin-IC | 160 | Water | ||
| 125 | DMF, DMAc | |||
| MβCD-Vanillin-IC | 160 | Water | ||
| 160 | DMF, DMAc | |||
| HPβCD-4-aminoazobenzene-IC | 130 | Water | Drug delivery, sensors | [119] |
| HPβCD-diclofenac | 45 | Ethanol | Fast dissolving solid complexes | [120] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costoya, A.; Concheiro, A.; Alvarez-Lorenzo, C. Electrospun Fibers of Cyclodextrins and Poly(cyclodextrins). Molecules 2017, 22, 230. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22020230
Costoya A, Concheiro A, Alvarez-Lorenzo C. Electrospun Fibers of Cyclodextrins and Poly(cyclodextrins). Molecules. 2017; 22(2):230. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22020230
Chicago/Turabian StyleCostoya, Alejandro, Angel Concheiro, and Carmen Alvarez-Lorenzo. 2017. "Electrospun Fibers of Cyclodextrins and Poly(cyclodextrins)" Molecules 22, no. 2: 230. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22020230