Ultrasound-Assisted Extraction and Identification of Natural Antioxidants from the Fruit of Melastoma sanguineum Sims
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single Factor Experiment Analysis
2.1.1. Effect of Ethanol Concentration
2.1.2. Effect of Solvent/Material Ratio
2.1.3. Effect of Extracting Time
2.1.4. Effect of Extracting Temperature
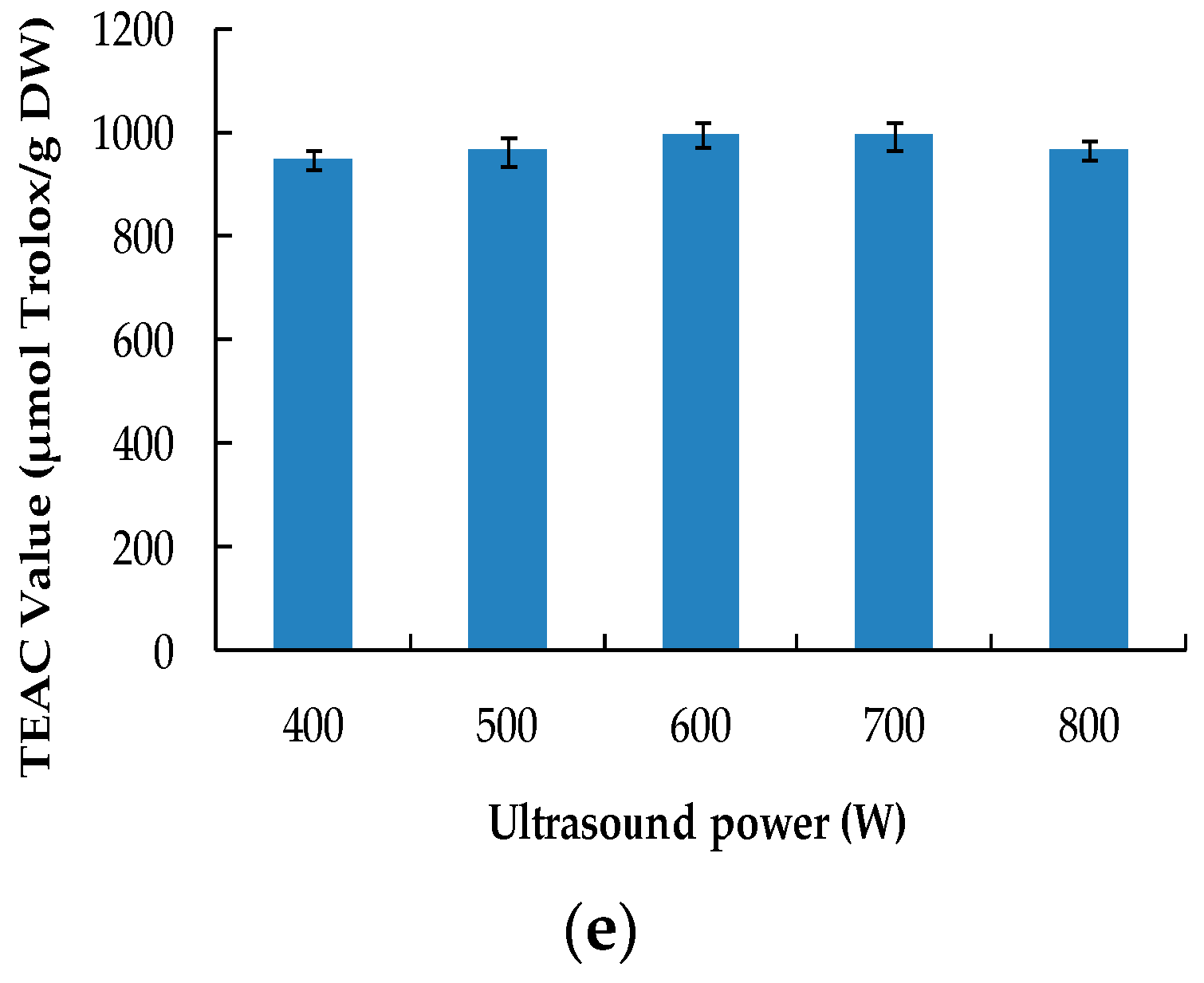
2.1.5. Effect of Ultrasound Power
2.2. Optimization by Response Surface Methodology
2.2.1. Experimental Design and Results
2.2.2. Fitting the Model
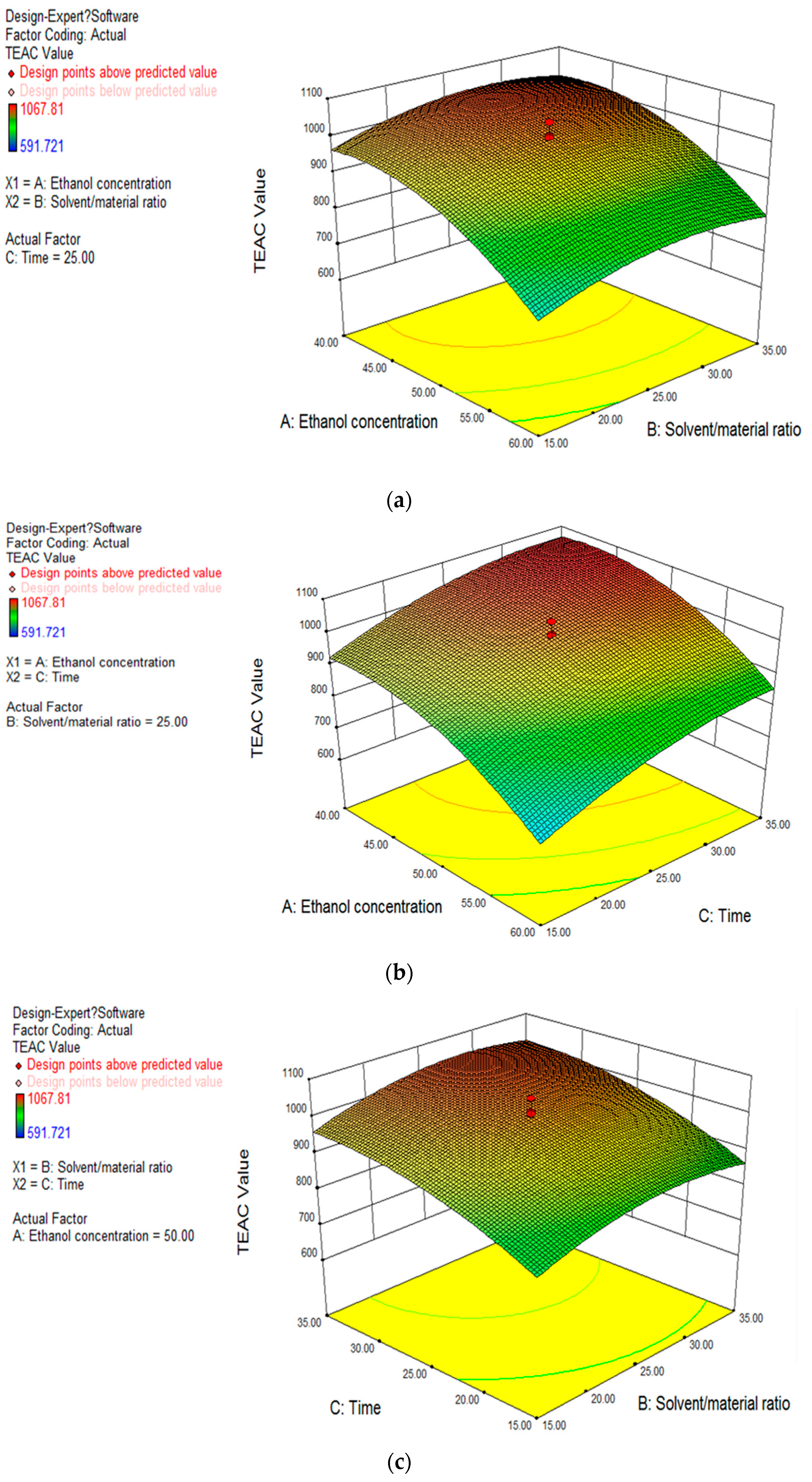
2.2.3. Response Surfaces Analysis
2.2.4. Validation of Predicted Value
2.3. Comparison of UAE with Conventional Extraction Methods
2.4. Identification and Quantification of Phenolic Components
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. Extraction of Natural Antioxidants
3.3.1. Ultrasound-Assisted Extraction
3.3.2. Maceration Extraction
3.3.3. Soxhlet Extraction
3.4. Experimental Design
3.4.1. Single Factor Experiment
3.4.2. Response Surface Methodology
3.5. Determination of Antioxidant Capacity
3.6. Determination of Total Phenolic Content
3.7. Determination of Total Flavonoid Content
3.8. Identification and Quantification of Phenolic Compounds
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lue, J.M.; Lin, P.H.; Yao, Q.Z.; Chen, C.Y. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Azizova, O.A. Role of free radical processes in the development of atherosclerosis. Biol. Membr. 2002, 19, 451–471. [Google Scholar]
- Santilli, F.; D’Ardes, D.; Davi, G. Oxidative stress in chronic vascular disease: From prediction to prevention. Vasc. Pharmacol. 2015, 74, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Nicklett, E.J.; Semba, R.D.; Xue, Q.L.; Tian, J.; Sun, K.; Cappola, A.R.; Simonsick, E.M.; Ferrucci, L.; Fried, L.P. Fruit and vegetable intake, physical activity, and mortality in older community-dwelling women. J. Am. Geriatr. Soc. 2012, 60, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Muller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H.; Nunez, M.J.; Parajo, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Song, F.L.; Gan, R.Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.B. Total phenolic contents and antioxidant capacities of selected Chinese medicinal plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, Y.Y.; Chao, L.F.; Wang, S.Q.; Wu, W.; Dai, S.P.; Wang, F.; Fan, Q.; Zhou, R.C. Extensive hybridization and introgression between Melastoma candidum and M-sanguineum. PLoS ONE 2014, 9, e96680. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Melastomataceae. In Flora Reipublicae Popularis Sinicae; Chen, C., Chang, H., Miau, R., Hsu, T., Eds.; Science Press: Beijing, China, 1984; Volume 53, pp. 152–162. [Google Scholar]
- Lee, I.; Kim, I.S.; Lee, Y.M.; Lee, Y.; Kim, J.; Kim, J.S. 2″,4″-o-diacetylquercitrin, a novel advanced glycation end-product formation and aldose reductase inhibitor from Melastoma sanguineum. Chem. Pharm. Bull. 2013, 61, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Xie, F. Chinese Medicine Useful for Treating Osteomyelitis and Inflammation, Comprises Embelia laeta, Melastoma sanguineum and Paeonia ostii. CN101919897-A; CN101919897-B. 22 December 2010. [Google Scholar]
- Fu, L.; Xu, B.T.; Xu, X.R.; Qin, X.S.; Gan, R.Y.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 wild fruits from south china. Molecules 2010, 15, 8602–8617. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Oliver, C.M.; Kentish, S.; Ashokkumar, M. Use of power ultrasound to improve extraction and modify phase transitions in food processing. Food Rev. Int. 2013, 29, 67–91. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.B.; Lu, X.H.; Cai, M.Q.; Xu, Z.C. Improvement of leaching process of geniposide with ultrasound. Ultrason. Sonochem. 2006, 13, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.-Q.; Ai, X.-X.; Zang, S.-Y.; Guan, T.-T.; Xu, X.-R.; Li, H.-B. Ultrasound-assisted extraction of phillyrin from Forsythia suspensa. Ultrason. Sonochem. 2011, 18, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Pico, Y. Ultrasound-assisted extraction for food and environmental samples. TrAC Trend Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Gan, C.Y.; Latiff, A.A. Optimization of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2011, 124, 1277–1283. [Google Scholar] [CrossRef]
- Esclapez, M.D.; Garcia-Perez, J.V.; Mulet, A.; Carcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Manrique, G.D.; Dimitrov, K. Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium quinoa) seeds. J. Food Sci. Technol. 2015, 52, 4396–4404. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, L.G.; Dimitrov, K.; Vauchel, P.; Nikov, I. Kinetics of ultrasound assisted extraction of anthocyanins from Aronia melanocarpa (black chokeberry) wastes. Chem. Eng. Res. Des. 2014, 92, 1818–1826. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimization of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, C.; Guener, A. Solubility profiles of poly(ethylene glycol)/solvent systems, I: Qualitative comparison of solubility parameter approaches. Eur. Polym. J. 2007, 43, 3068–3093. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, S.Y. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.). Ultrason. Sonochem. 2013, 20, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.N.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatropha integerrima by response surface methodology. Molecules 2016, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Clarification of high-added value products from olive mill wastewater. J. Food Eng. 2010, 99, 190–197. [Google Scholar] [CrossRef]
- Yue, T.L.; Shao, D.Y.; Yuan, Y.H.; Wang, Z.L.; Qiang, C.Y. Ultrasound-assisted extraction, HPLC analysis, and antioxidant activity of polyphenols from unripe apple. J. Sep. Sci. 2012, 35, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Both, S.; Chemat, F.; Strube, J. Extraction of polyphenols from black tea—Conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2014, 21, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Tabaraki, R.; Rastgoo, S. Comparison between conventional and ultrasound-assisted extractions of natural antioxidants from walnut green husk. Korean J. Chem. Eng. 2014, 31, 676–683. [Google Scholar] [CrossRef]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Q.; Ye, X.Q.; Fang, Z.X.; Chen, J.C.; Xu, G.H.; Liu, D.H. Phenolic compounds and antioxidant activity of extracts from ultrasonic treatment of satsuma mandarin (Citrus unshiu Marc.) peels. J. Agric. Food Chem. 2008, 56, 5682–5690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.G.; Cao, Y.P.; Tian, Y.A.; Li, X.H. Optimization of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Food. 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Sakakibara, H.; Honda, Y.; Nakagawa, S.; Ashida, H.; Kanazawa, K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003, 51, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, P.; Bomzan, D.P.; Rao, B.V.S.; Sreerama, Y.N. Distribution of phenolic antioxidants in whole and milled fractions of quinoa and their inhibitory effects on alpha-amylase and alpha-glucosidase activities. Food Chem. 2016, 199, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Extraction of natural antioxidants from the Thelephora ganbajun mushroom by an ultrasound-assisted extraction technique and evaluation of antiproliferative activity of the extract against human cancer cells. Int. J. Mol. Sci. 2016, 17, 1664. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Tsagiannidis, A.; Sarakatsianos, I. Simultaneous determination of polyphenols and major purine alkaloids in greek sideritis species, herbal extracts, green tea, black tea, and coffee by high-performance liquid chromatography-diode array detection. J. Sep. Sci. 2012, 35, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Pan, M.H.; Chiou, Y.S.; Wang, Y.J.; Ho, C.T.; Lin, J.K. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011, 2, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. 2015, 72, 643–650. [Google Scholar] [PubMed]
- Upadhyay, R.; Rao, L.J.M. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.A.; Amit, T.; Kalfon, L.; Reznichenko, L.; Weinreb, O.; Youdim, M.B.H. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: Special reference to epigallocatechin gallate (EGCG). J. Alzheimers Dis. 2008, 15, 211–222. [Google Scholar]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Huttemann, M. Molecular mechanisms and therapeutic effects of (−)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Investig. 2014, 37, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.L.; Li, H. Recovery and purification of cholesterol from cholesterol-beta-cyclodextrin inclusion complex using ultrasound-assisted extraction. Ultrason. Sonochem. 2017, 34, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Xu, D.P.; Li, S.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from sugar apple (Annona squamosa L.) peel using response surface methodology. Molecules 2015, 20, 20448–20459. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kalia, K.; Sharma, K.; Singh, H.P.; Singh, B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea lodd and quantification of its phenolic constituents by RP-HPLC. J. Agric. Food Chem. 2008, 56, 10129–10134. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.

| Run | X1 (Ethanol Concentration, %) | X2 (Solvent/Material Ratio, mL/g) | X3 (Extracting Time, min) | Y (TEAC Value, μmol Trolox/g DW) | |
|---|---|---|---|---|---|
| Actual Value | Predicted Value | ||||
| 1 | 50 | 25 | 25 | 988.49 | 998.34 |
| 2 | 50 | 25 | 25 | 981.79 | 998.34 |
| 3 | 40 | 35 | 15 | 876.92 | 905.11 |
| 4 | 60 | 35 | 35 | 855.01 | 846.94 |
| 5 | 40 | 35 | 35 | 1067.81 | 1050.23 |
| 6 | 66.82 | 25 | 25 | 591.72 | 604.16 |
| 7 | 50 | 25 | 25 | 1005.26 | 998.34 |
| 8 | 33.18 | 25 | 25 | 999.67 | 975.19 |
| 9 | 50 | 25 | 8.18 | 789.55 | 758.87 |
| 10 | 50 | 41.82 | 25 | 911.31 | 921.25 |
| 11 | 50 | 25 | 25 | 1045.49 | 998.34 |
| 12 | 60 | 15 | 35 | 799.64 | 779.95 |
| 13 | 50 | 25 | 41.82 | 997.43 | 1016.08 |
| 14 | 50 | 8.18 | 25 | 846.54 | 824.57 |
| 15 | 50 | 25 | 25 | 1008.61 | 998.34 |
| 16 | 60 | 35 | 15 | 689.15 | 676.78 |
| 17 | 60 | 15 | 15 | 593.10 | 619.19 |
| 18 | 50 | 25 | 25 | 958.32 | 998.34 |
| 19 | 40 | 15 | 35 | 971.98 | 992.86 |
| 20 | 40 | 15 | 15 | 840.55 | 857.13 |
| validation | 42.98 | 28.29 | 34.29 | 1074.61 | 1077.37 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 368,043.36 | 9 | 40,893.71 | 40.59 | <0.0001 | significant |
| X1(Ethanol concentration) | 166,172.90 | 1 | 166,172.90 | 164.93 | <0.0001 | |
| X2 (Solvent/material ratio) | 11,282.75 | 1 | 11,282.75 | 11.20 | 0.0074 | |
| X3 (Extracting time) | 79,864.25 | 1 | 79,864.25 | 79.27 | <0.0001 | |
| X1X2 | 46.19 | 1 | 46.19 | 0.05 | 0.8348 | |
| X1X3 | 313.39 | 1 | 313.39 | 0.31 | 0.5893 | |
| X2X3 | 44.07 | 1 | 44.07 | 0.04 | 0.8385 | |
| X12 | 78,430.22 | 1 | 78,430.22 | 77.84 | <0.0001 | |
| X22 | 28,340.63 | 1 | 28,340.63 | 28.13 | 0.0003 | |
| X32 | 22,140.13 | 1 | 22,140.13 | 21.97 | 0.0009 | |
| Residual | 10,075.50 | 10 | 1007.55 | |||
| Lack of Fit | 5726.49 | 5 | 1145.30 | 1.32 | 0.3850 | Not significant |
| Pure Error | 4349.02 | 5 | 869.80 | |||
| Cor Total | 378,118.86 | 19 | ||||
| R-Squared | 0.973 | |||||
| Adj R-Squared | 0.949 |
| Extracting Methods | Ethanol Concentration | Time | Temperature | TEAC Value (μmol Trolox/g DW) | TPC (mg GAE/g DW) | TFC (mg QE/g DW) |
|---|---|---|---|---|---|---|
| Maceration | 42.98% | 24 h | 25 °C | 723.27 ± 11.61 | 104.21 ± 2.17 | 1.39 ± 0.07 |
| Soxhlet | 42.98% | 4 h | 95 °C | 518.37 ± 23.23 | 76.05 ± 2.71 | 1.89 ± 0.16 |
| UAE | 42.98% | 34.29 min | 60 °C | 1074.61 ± 32.56 | 158.61 ± 6.44 | 2.58 ± 0.18 |
| Phenolic Components | Retention Time (tR, min) | Parent Ion (m/z, [M − H]¯) | Product Ion (m/z) | Contents (μg/g DW) |
|---|---|---|---|---|
| epicatechin gallate | 6.87 | 441 | 169 | 1019.57 ± 99.39 |
| epicatechin | 5.4 | 289 | 203 | 90.34 ± 4.84 |
| rutin | 9.67 | 609 | 300 | 61.6 ± 1.59 |
| epigallocatechin | 3.03 | 305 | 137 | 28.67 ± 2.62 |
| protocatechuic acid | 3.09 | 152.9 | 107.8 | 3.22 ± 0.23 |
| chlorogenic acid | 4.13 | 353 | 191 | 2.34 ± 0.28 |
| quercetin | 11.8 | 301 | 179 | 1.39 ± 0.18 |
| Independent Variable | Units | Symbol | Coded Levels | ||||
|---|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | |||
| Ethanol concentration | % (v/v) | X1 | 33.18 | 40 | 50 | 60 | 66.82 |
| Solvent/material ratio | mL/g | X2 | 8.18 | 15 | 25 | 35 | 41.82 |
| Extracting time | min | X3 | 8.18 | 15 | 25 | 35 | 41.82 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Xu, D.-P.; Lin, S.-J.; Li, Y.; Zheng, J.; Zhou, Y.; Zhang, J.-J.; Li, H.-B. Ultrasound-Assisted Extraction and Identification of Natural Antioxidants from the Fruit of Melastoma sanguineum Sims. Molecules 2017, 22, 306. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22020306
Zhou T, Xu D-P, Lin S-J, Li Y, Zheng J, Zhou Y, Zhang J-J, Li H-B. Ultrasound-Assisted Extraction and Identification of Natural Antioxidants from the Fruit of Melastoma sanguineum Sims. Molecules. 2017; 22(2):306. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22020306
Chicago/Turabian StyleZhou, Tong, Dong-Ping Xu, Sheng-Jun Lin, Ya Li, Jie Zheng, Yue Zhou, Jiao-Jiao Zhang, and Hua-Bin Li. 2017. "Ultrasound-Assisted Extraction and Identification of Natural Antioxidants from the Fruit of Melastoma sanguineum Sims" Molecules 22, no. 2: 306. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22020306






