Briarenols C–E, New Polyoxygenated Briaranes from the Octocoral Briareum excavatum
Abstract
:1. Introduction
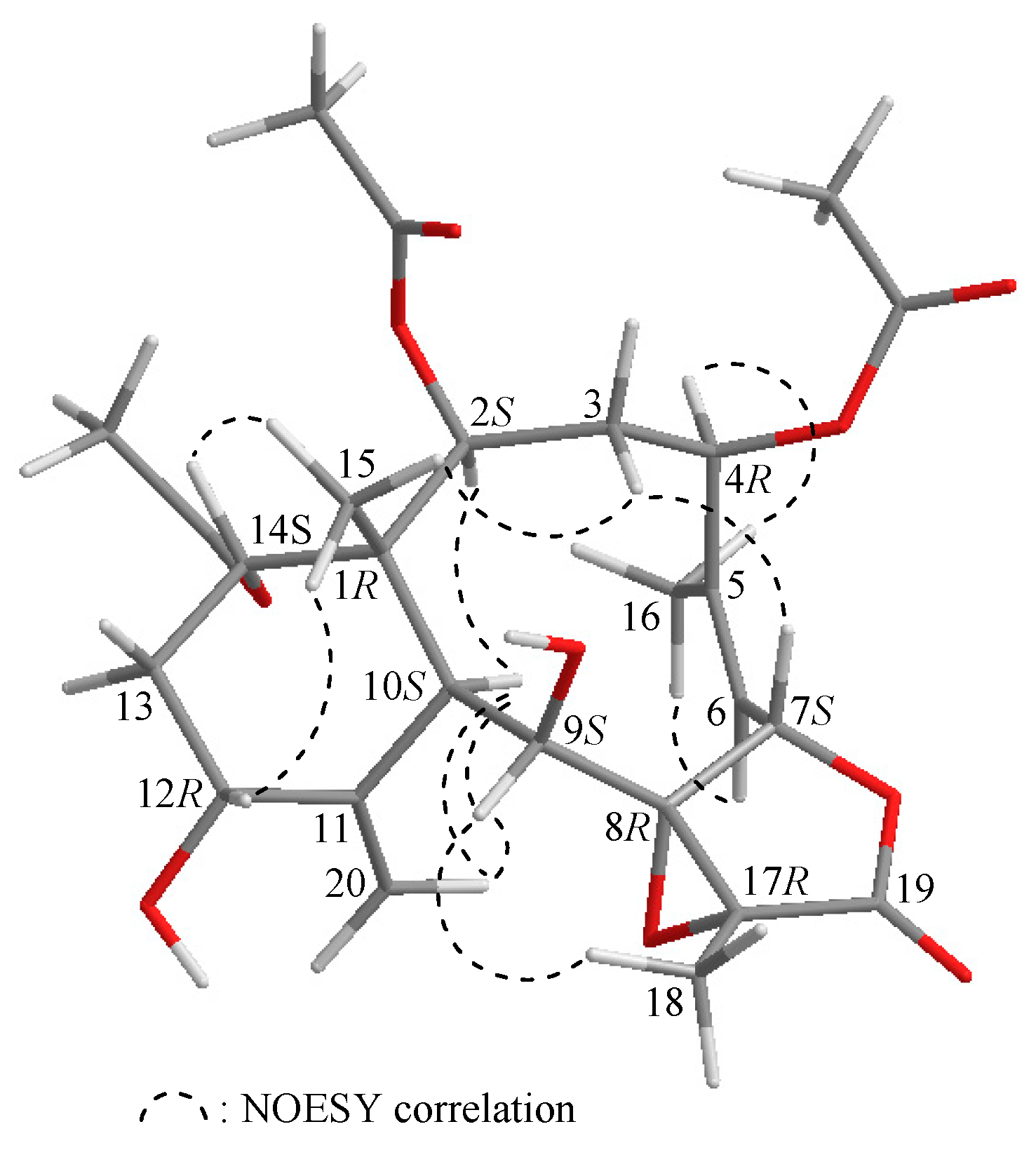
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Generation of Superoxide Anions and Release of Elastase by Human Neutrophils
3.5. In Vitro Anti-Inflammatory Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sung, P.-J.; Sheu, J.-H.; Xu, J.-P. Survey of briarane-type diterpenoids of marine origin. Heterocycles 2002, 57, 535–579. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chang, P.-C.; Fang, L.-S.; Sheu, J.-H.; Chen, W.-C.; Chen, Y.-P.; Lin, M.-R. Survey of briarane-related diterpenoids: Part II. Heterocycles 2005, 65, 195–204. [Google Scholar] [CrossRef]
- Sung, P.-J.; Sheu, J.-H.; Wang, W.-H.; Fang, L.-S.; Chung, H.-M.; Pai, C.-H.; Su, Y.-D.; Tsai, W.-T.; Chen, B.-Y.; Lin, M.-R. Survey of briarane-related diterpenoids: Part III. Heterocycles 2008, 75, 2627–2648. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, J.-H.; Wang, W.-H.; Sheu, J.-H.; Fang, L.-S.; Wu, Y.-C.; Chen, Y.-H.; Chung, H.-M.; Su, Y.-D.; Chang, Y.-C. Survey of briarane-related diterpenoids: Part IV. Heterocycles 2011, 83, 1241–1258. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Chen, Y.-H.; Chen, Y.-H.; Su, Y.-D.; Chang, Y.-C.; Su, J.-H.; Weng, C.-F.; Lee, C.-H.; Fang, L.-S.; Wang, W.-H. Briarane diterpenoids isolated from gorgonian corals between 2011 and 2013. Mar. Drugs 2014, 12, 2164–2181. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Su, J.-H.; Hwang, T.-L.; Wen, Z.-H.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Briarane diterpenoids isolated from octocorals between 2014 and 2016. Mar. Drugs 2017, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- González, Y.; Torres-Mendoza, D.; Jones, G.E.; Fernandez, P.L. Marine diterpenoids as potential anti-inflammatory agents. Mediat. Inflamm. 2015, 2015, 263543. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-C.; Sung, P.-J.; Duh, C.-Y.; Chen, B.-W.; Sheu, J.-H.; Yang, N.-S. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs 2013, 11, 4083–4126. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Kim, S.-K. Marine invertebrate natural products for anti-inflammatory and chronic disease. Evid. Based Complement. Altern. Med. 2013, 2013, 572859. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Wang, S.-H.; Chiang, M.Y.; Su, Y.-D.; Chang, Y.-C.; Hu, W.-P.; Tai, C.-Y.; Liu, C.-Y. Discovery of new dhlorinated briaranes from Junceella fragilis. Bull. Chem. Soc. Jpn. 2009, 82, 1426–1432. [Google Scholar] [CrossRef]
- Burks, J.E.; van der Helm, D.; Chang, C.Y.; Ciereszko, L.S. The crystal and molecular structure of briarein A, a diterpenoid from the gorgonian Briareum asbestinum. Acta Cryst. 1977, B33, 704–709. [Google Scholar] [CrossRef]
- Su, Y.-D.; Sung, C.-S.; Wen, Z.-H.; Chen, Y.-H.; Chang, Y.-C.; Chen, J.-J.; Fang, L.-S.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. New 9-hydroxybriarane diterpenoids from a gorgonian coral Briareum sp. Int. J. Mol. Sci. 2016, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Samimi-Namin, K.; van Ofwegen, L.P. Overview of the genus Briareum (Cnidaria, Octocorallia, Briareidae) in the Indo-Pacific, with the description of a new species. ZooKeys 2016, 557, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Chung, P.-J.; Ho, C.-M.; Kuo, C.-Y.; Hung, M.-F.; Huang, Y.-T.; Chang, W.-Y.; Chang, Y.-W.; Chan, K.-H.; Hwang, T.-L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenzamido) benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Chen, N.-F.; Chen, W.-F.; Hung, H.-C.; Lee, H.-P.; Lin, Y.-Y.; Wang, H.-M.; Sung, P.-J.; Sheu, J.-H.; Wen, Z.-H. Sinularin from indigenous soft coral attenuates nociceptive responses and spinal neuroinflammation in carrageenan-induced inflammatory rat model. Mar. Drugs 2012, 10, 1899–1919. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.-H.; Chen, W.-F.; Sung, C.-S.; Duh, C.-Y.; Huang, S.-Y.; Lin, C.-S.; Tai, M.-H.; Tzeng, S.-F.; Wen, Z.-H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 2009, 158, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.-H.; Chen, W.-F.; Duh, C.-Y.; Huang, S.-Y.; Hsu, C.-H.; Lin, C.-S.; Sung, C.-S.; Chen, I.-M.; Wen, Z.-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–3 are not available from the authors.

| Position | δH (J in Hz) | δC, Multiple | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 47.1, C | |||
| 2 | 4.80 d (4.4) | 72.0, CH | H2-3 | C-1, -4, -15, acetate carbonyl |
| 3α/β | 1.96 m; 3.15 dd (12.8, 11.6) | 37.7, CH2 | H-2, H-4 | C-1, -2, -4, -5 |
| 4 | 5.19 dd (12.8, 6.0) | 73.0, CH | H2-3 | C-3, -6, -16 |
| 5 | 144.8, C | |||
| 6 | 5.59 d (10.0) | 123.4, CH | H-7, H3-16 | C-8 |
| 7 | 5.94 d (10.0) | 73.2, CH | H-6 | C-5 |
| 8 | 71.4, C | |||
| 9 | 3.95 br s | 72.3, CH | H-10 | C-17 |
| 10 | 3.39 d (5.2) | 44.4, CH | H-9 | C-1, -2, -9, -11, -12, -15, -20 |
| 11 | 150.6, C | |||
| 12 | 4.51 dd (8.8, 7.6) | 65.5, CH | H2-13 | n.o. a |
| 13α/β | 1.55 m; 2.62 ddd (16.0, 8.8, 4.4) | 37.2, CH2 | H-12, H-14 | C-1, -11, -12, -14 |
| 14 | 4.74 d (4.4) | 74.1, CH | H2-13 | C-1, -10, -12, acetate carbonyl |
| 15 | 1.34 s | 16.4, CH3 | C-1, -2, -10, -14 | |
| 16 | 2.20 d (1.2) | 25.6, CH3 | H-6 | C-4, -5, -6 |
| 17 | 60.6, C | |||
| 18 | 1.53 s | 9.5, CH3 | C-8, -17, -19 | |
| 19 | 171.9, C | |||
| 20a | 5.13 d (1.2) | 109.3, CH2 | H-20b | C-10, -12 |
| b | 5.03 s | H-20a | C-10, -12 | |
| OAc-2 | 170.6, C | |||
| 2.01 s | 20.9, CH3 | Acetate carbonyl | ||
| OAc-4 | 170.3, C | |||
| 1.91 s | 21.1, CH3 | Acetate carbonyl | ||
| OAc-14 | 170.3, C | |||
| 2.04 s | 21.0, CH3 | Acetate carbonyl |
| Position | δH (J in Hz) | δC, Multiple | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 48.0, C | |||
| 2 | 5.01 d (7.2) | 72.5, CH | H2-3 | C-1, -3, -4, -10, -15, acetate carbonyl |
| 3α/β | 2.00 m; 3.07 dd (15.2, 12.4) | 37.6, CH2 | H-2, H-4 | C-1, -2, -4, -5 |
| 4 | 5.08 dd (12.4, 5.6) | 72.8, CH | H2-3 | C-3, -5, -6, -16, acetate carbonyl |
| 5 | 143.6, C | |||
| 6 | 5.47 ddd (9.6, 1.2, 1.2) | 123.4, CH | H-7, H3-16 | C-4, -16 |
| 7 | 5.91 d (9.6) | 74.2, CH | H-6 | C-5, -6, -19 |
| 8 | 71.2, C | |||
| 9 | 4.35 br s | 73.4, CH | H-10, OH-9 | C-8, -10, -11 |
| 10 | 2.96 d (2.8) | 44.1, CH | H-9 | C-1, -2, -8, -9, -11, -12, -14, -15, -20 |
| 11 | 151.5, C | |||
| 12 | 4.31 dd (6.8, 6.8) | 69.7, CH | H2-13 | C-10, -11, -13, -14, -20 |
| 13α/β | 2.19 ddd (15.2, 6.8, 3.2); 1.81 ddd (15.2, 6.8, 3.6) | 36.6, CH2 | H-12, H-14 | C-1, -11, -12, -14 |
| 14 | 4.76 dd (3.6, 3.2) | 73.8, CH | H2-13 | C-10, -12 |
| 15 | 1.30 s | 14.7, CH3 | C-1, -2, -10, -14 | |
| 16 | 2.11 d (1.2) | 25.4, CH3 | H-6 | C-4, -5, -6 |
| 17 | 62.2, C | |||
| 18 | 1.52 s | 10.0, CH3 | C-8, -17, -19 | |
| 19 | 172.0, C | |||
| 20a | 5.29 s | 110.9, CH2 | H-20b | C-10, -11, -12 |
| b | 5.07 s | H-20a | C-10, -11, -12 | |
| OAc-2 | 170.4, C | |||
| 2.01 s | 21.0, CH3 | Acetate carbonyl | ||
| OAc-4 | 170.3, C | |||
| 2.04 s | 21.0, CH3 | Acetate carbonyl | ||
| OAc-14 | 170.6, C | |||
| 1.96 s | 21.3, CH3 | Acetate carbonyl | ||
| OH-9 | 3.00 d (4.8) | H-9 |
| Position | δH (J in Hz) | δC, Multiple | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 45.3, C | |||
| 2 | 4.99 d (6.4) | 73.9, CH | H2-3 | C-1, -3, -10, -14, -15, acetate carbonyl |
| 3α/β | 1.34 dd (15.6, 4.8); 3.37 ddd (15.6, 12.8, 6.4) | 35.8, CH2 | H-2, H-4 | C-1, -2, -4, -5 |
| 4 | 4.80 dd (12.8, 4.8) | 76.3, CH | H2-3 | C-2, -3, -6, -8, -16 |
| 5 | 138.0, C | |||
| 6 | 5.51 ddd (2.8, 2.4, 2.4) | 55.1, CH | H-7, H2-16 | C-5, -16 |
| 7 | 4.74 d (2.8) | 80.5, CH | H-6 | C-5, -6 |
| 8 | 82.0, C | |||
| 9 | 4.87 d (3.6) | 76.3, CH | OH-9 | C-1, -8, -10, -11, -17 |
| 10 | 2.16 s | 40.4, CH | n.o. a | C-1, -2, -8, -9, -11, -12, -14, -15 |
| 11 | 78.4, C | |||
| 12 | 3.49 m | 76.2, CH | H2-13, OH-12 | n.o. |
| 13α/β | 1.96 ddd (15.6, 3.6, 2.8); 2.43 ddd (15.6, 4.0, 2.8) | 28.0, CH2 | H-12, H-14 | C-1, -12, -14 |
| 14 | 5.18 dd (2.8, 2.8) | 76.4, CH | H2-13 | C-2, -10, -12, acetate carbonyl |
| 15 | 1.53 s | 16.6, CH3 | C-1, -2, -10, -14 | |
| 16a | 5.29 d (2.4) | 115.7, CH2 | H-6, H-16b | C-4, -6 |
| b | 5.46 d (2.4) | H-6, H-16a | C-4, -5, -6 | |
| 17 | 2.59 q (7.2) | 50.2, CH | H3-18 | C-8, -9, -18, -19 |
| 18 | 1.29 d (7.2) | 8.2, CH3 | H-17 | C-8, -17, -19 |
| 19 | 175.9, C | |||
| 20 | 1.54 s | 29.5, CH3 | C-10, -11, -12 | |
| OAc-2 | 170.8, C | |||
| 1.99 s | 21.2, CH3 | Acetate carbonyl | ||
| OAc-14 | 169.2, C | |||
| 2.04 s | 21.1, CH3 | Acetate carbonyl | ||
| OH-9 | 2.78 d (3.6) | H-9 | C-8, -9 | |
| OH-12 | 2.71 d (9.2) | H-12 |
| Compound | Superoxide Anions | Elastase Release |
|---|---|---|
| IC50 (μM) a | IC50 (μM) | |
| 1 | >10 | >10 |
| 2 | >10 | 4.65 ± 1.50 |
| 3 | >10 | >10 |
| LY294002 b | 1.39 ± 0.32 | 3.30 ± 0.11 |
| Compound | iNOS | COX-2 |
|---|---|---|
| Expression (% of LPS Group) | Expression (% of LPS Group) | |
| Control | 0.79 ± 0.01 | 1.00 ± 0.02 |
| LPS | 100.00 ± 7.48 | 100.00 ± 18.39 |
| 1 | 93.22 ± 22.59 | 100.41 ± 1.08 |
| 2 | 78.35 ± 0.73 | 94.28 ± 21.35 |
| 3 | 66.86 ± 3.86 | 119.42 ± 1.33 |
| DEX a | 56.18 ± 4.53 | 17.42 ± 2.53 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.-F.; Su, Y.-D.; Hwang, T.-L.; Liao, Z.-J.; Tsui, K.-H.; Wen, Z.-H.; Wu, Y.-C.; Sung, P.-J. Briarenols C–E, New Polyoxygenated Briaranes from the Octocoral Briareum excavatum. Molecules 2017, 22, 475. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22030475
Chen N-F, Su Y-D, Hwang T-L, Liao Z-J, Tsui K-H, Wen Z-H, Wu Y-C, Sung P-J. Briarenols C–E, New Polyoxygenated Briaranes from the Octocoral Briareum excavatum. Molecules. 2017; 22(3):475. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22030475
Chicago/Turabian StyleChen, Nan-Fu, Yin-Di Su, Tsong-Long Hwang, Zuo-Jian Liao, Kuan-Hao Tsui, Zhi-Hong Wen, Yang-Chang Wu, and Ping-Jyun Sung. 2017. "Briarenols C–E, New Polyoxygenated Briaranes from the Octocoral Briareum excavatum" Molecules 22, no. 3: 475. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22030475






