Green Synthesis of Ag/Ag2O Nanoparticles Using Aqueous Leaf Extract of Eupatorium odoratum and Its Antimicrobial and Mosquito Larvicidal Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Preparation of Aqueous Leaf Extract of E. odoratum
2.3. Synthesis of Silver/Silver Oxide Nanoparticles
2.4. Characterization of the Synthesized Nanoparticles
2.5. Mosquito Larvicidal Efficacy
2.5.1. Collection of Mosquito Larvae
2.5.2. Mosquito Larvicidal Assay
2.5.3. Dose Response Bioassay
2.6. Antimicrobial Studies
2.6.1. Antimicrobial Assay
2.6.2. Minimum Inhibitory Concentration (MIC) Studies for the Nanoparticles
3. Results and Discussion
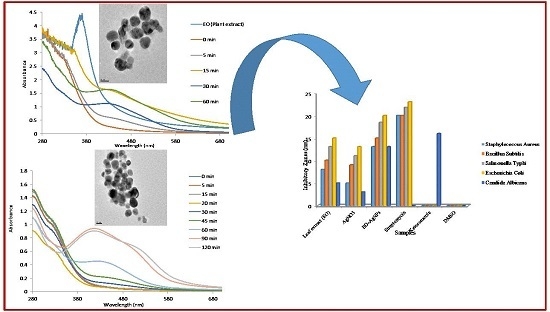
3.1. UV-VISible Spectral Studies
3.2. Transmission Electron Microscopy (TEM) Analysis
3.3. Powder X-ray Diffraction (PXRD) Results
3.4. Mosquito Larvicidal Studies
3.5. Antimicrobial Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hussain, I.; Brust, M.; Papworth, A.J.; Cooper, A.I. Preparation of Acrylate-Stabilized Gold and Silver Hydrosols and Gold-Polymer Composite Films. Langmuir 2003, 19, 4831–4835. [Google Scholar] [CrossRef]
- Sharma, V.K.; Ria, A.Y.; Yekaterina, L. Silver nanoparticles: Green synthesis and their antimicrobial activities. J. Colloid Interf. Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, S.; Aniket, G.; Mahendra, R. Large scale synthesis and antibacterial activity of fungal derived silver nanoparticles. Environ. Chem. Lett. 2016. [Google Scholar] [CrossRef]
- Pulit, J.; Banach, M. Preparation of nanosilver and nanogold based on Dog Rose aqeous extract. Bioinorg. Chem. Appl. 2014, 2014, 658935. [Google Scholar] [CrossRef] [PubMed]
- Shalaka, A.M.; Pratik, R.C.; Vrishali, B.S.; Suresh, P.K. Rapid biosynthesis of silver nanoparticles using Cymbopogan citratus (Lemongrass) and its antimicrobial activity. Nano-Micro Lett. 2011, 3, 189–194. [Google Scholar]
- Gardea-Torresdey, J.L.; Parsons, J.G.; Gomez, E.; Peralta-Videa, J.; Troiani, H.E.; Santiago, P.; Yacaman, M.J. Formation and Growth of Au Nanoparticles inside Live Alfalfa Plants. Nano Lett. 2002, 2, 397–401. [Google Scholar] [CrossRef]
- Monaliben, S.; Derek, F.; Shashi, S.; Suraj, K.T.; Gérrard, E.J.P. Green synthesis of metal nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar]
- Baldi, F.; Daniele, S.; Gallo, M.; Paganelli, S.; Battistel, D.; Piccolo, O.; Faleri, C.; Puglia, A.M.; Gallo, G. Polysaccharide-based silver nanoparticles synthesized by Klebsiella oxytoca DCA 29614 cause DNA fragmentation in E. coli cells. Biometals 2016, 29, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Southam, G.; Beveridge, T.J. The in vitro formation of placer gold by bacteria. Geochim. Cosmochim. Acta 1994, 58, 4527–4530. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, L.A.M.; Paz Lima, M.; Marques, M.O.M.; Facanali, R.; Silva Pinto, A.C.; Pedro Tadei, W. Chemical Composition and Larvicidal activity against Aedes aegypti Larvae of Essential Oils from Four Guarea Species. Molecules 2010, 15, 5734–5741. [Google Scholar] [CrossRef] [PubMed]
- Macoris, M.L.G.; Andrighetti, M.T.M.; Takaku, L.; Glasser, C.M.; Garbeloto, V.C.; Bracco, J.E. Resistance of Aedes aegypti from the state of Sao Paulo, Brazil, to organophosphates insecticides. Mem. Inst. Oswaldo Cruz. 2003, 98, 703–708. [Google Scholar] [CrossRef]
- Meenakshi, S.V.; Jayaprakash, K. Mosquito larvicidal efficacy of leaf extract from mangrove plant Rhizophora mucronata (Family: Rhizophoraceae) against Anopheles and Aedes species. J. Pharm. Phytochem. 2014, 3, 78–83. [Google Scholar]
- Ramamurthy, V.; Krishnaveni, S. Larvicidal efficacy of leaf extracts of Heliotropium Indicum and Mukia maderaspatana against the dengue fever mosquito vector Aedes aegypti. J. Entomol. Zool. Stud. 2014, 2, 40–45. [Google Scholar]
- Banu, A.N.; Balasubramanian, C. Myco-synthesis of silver nanoparticles using Beauveria bassiana against dengue vector, Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2014, 113, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Banu, A.N.; Balasubramanian, C. Optimization and synthesis of silver nanoparticles using Isaria fumosorosea against human vector mosquitoes. Parasitol. Res. 2014, 113, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Suganya, G.; Karthi, S.; Shivakumar, M.S. Larvicidal potential of silver nanoparticles synthesized from Leucas aspera leaf extracts against dengue vector Aedes aegypti. Parasitol. Res. 2014, 113, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.A.; Evan, C.W.; Raston, C.L. Green chemistry and the health implications of nanoparticles. Green Chem. 2006, 8, 417–432. [Google Scholar] [CrossRef]
- Veerakumar, K.; Govindarajan, M.; Rajeswary, M. Green synthesis of silver nanoparticles using Sida acuta (Malvaceae) leaf extract against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2013, 112, 4073–4085. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, K.; Govindarajan, M.; Rajeswary, M.; Muthukumaran, U. Low-cost and eco-friendly green synthesis of silver nanoparticles using Feronia elephantum (Rutaceae) against Culex quinquefasciatus, Anopheles stephensi, and Aedesaegypti (Diptera: Culicidae). Parasitol. Res. 2014, 113, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Prakash, S. Silver nanoparticles: A possibility for malarial and filarial vector control technology. Parasitol. Res. 2014, 113, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.R.; Nattuthurai, N.; Gopinath, P.; Mariappan, T. Synthesis of eco-friendly silver nanoparticles from Morindatinctoria leaf extract and its larvicidal activity against Culexquinquefasciatus. Parasitol. Res. 2014, 114, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.C.; Leite, J.A.; Oliveira, L.H.G. The larvicidal activity of Agave sisalana against L4 larvae of Aedes aegyptis mediated by internal necrosis and inhibition of nitric oxide production. Parasitol. Res. 2015, 114, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Abdul Rahuman, A. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop. 2011, 118, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Velayutham, K.; Rahuman, A.A.; Rajakumar, G.; Roopan, S.M.; Elango, G.; Kamaraj, C.; Marimuthu, S.; Santhoshkumar, T.; Iyappan, M.; Siva, C. Larvicidal activity of green synthesized silver nanoparticles using bark aqueous extract of Ficus racemosa against Culex quinquefasciatus and Culex gelidus. Asian Pac. J. Trop. Med. 2013, 6, 95–101. [Google Scholar] [CrossRef]
- Rajasekharreddy, P.; Rani, P.U. Biofabrication of Ag nanoparticles using Sterculia foetida L. seed extract and their toxic potential against mosquito vectors and HeLa cancer cells. Mater. Sci. Eng. C 2014, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Akinmoladun, A.C.; Ibukun, E.O.; Danologe, I.A. Phytochemical constituents and antioxidant properties of extracts from the leaves of Chromolaena odorata. Sci. Res. Essays 2007, 2, 191–194. [Google Scholar]
- Owoyele, V.B.; Adediji, J.O.; Soladoye, A.O. Anti-inflammatory activity of aqueous leaf extract of Chromolaena odorata. Inflammopharmacology 2005, 13, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, O.B.; Olajide, O.A.; Soyannwo, O.O.; Makinde, J.M. Anti-inflammatory, antipyretic and antispasmodic: Properties of Chromolaena odorata. Pharm. Biol. 2000, 38, 367–370. [Google Scholar] [CrossRef]
- Cáceres, A.; Menéndez, H.; Méndez, E. Antigonorrhoeal activity of plants used in Guatemala for the treatment of sexually transmitted diseases. J. Ethnopharmacol. 1995, 48, 85–88. [Google Scholar] [CrossRef]
- Gopinath, R.; Sunilson, J.A.J.; Radhamani, S.; Das, A.; Nilugal, K. Diuretic activity of Eupatorium odoratum Linn. J. Pharm. Res. 2009, 2844–2846. [Google Scholar]
- Chomnawang, M.T.; Surassmo, S.; Nukoolkarn, V.S.; Gritsanapan, W. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J. Ethnopharmacol. 2005, 101, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.K.; Chakraborty, A.K. Evaluation of analgesic activity studies of various extracts of leaves of Eupatorium Odoratum Linn. Int. J. Pharm. Technol. 2010, 2, 612–616. [Google Scholar]
- Biswal, P.R.; Sardar, K.K.; Parija, S.C.; Mishra, P.R.; Misra, S.N. Wound healing effect of Eupatorium odoratum Linn. and Himax in rabbits. Indian J. Indig. Med. 1997, 19, 71–74. [Google Scholar]
- Morton, J.F. Atlas of Medicinal Plants of Middle America; Charles C. Thomas: Springfield, IL, USA, 1981; Volume 2. [Google Scholar]
- Adjanohoun, E.; Ake-Assi, L. Contribution Au Recensement des Plantes Medicinale de Cote D’Ivoire; Centre National de Floristique: Abidjan, Ivory Coast, 1979. [Google Scholar]
- Vijayaraghavan, K.; Mohamed, A.S.; Maruthi, R. Studies on phytochemical screening and antioxidant activity of Chromolaena odorata and Annona squamosa. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 7315–7320. [Google Scholar]
- Owolabi, M.S.; Ogundajo, A.; Yusuf, K.O. Chemical composition and bioactivity of the essential oil of Chromolaena odorata from Nigeria. Rec. Nat. Prod. 2010, 4, 72–78. [Google Scholar]
- Félicien, A.; Alain, A.G.; Sébastien, D.T. Chemical composition and Biological activities of the Essential oil extracted from the Fresh leaves of Chromolaena odorata (L. Robinson) growing in Benin. J. Biol. Sci. 2012, 1, 7–13. [Google Scholar]
- Lamaty, G.; Menut, C.; Zollo, P.H.A. Aromatic plants of tropical central Africa. IV. Essential oils of Eupatorium odoratum from Cameroon and Congo. J. Essent. Oil Res. 1992, 4, 101–105. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical composition of the essential oils of aerial parts and flowers of Chromolaena odorata (L.) R.M. King & H. Rob. from Western Ghats region of North West Karnataka. Indian J. Essent. Oil Bear. Plants 2013, 16, 71–75. [Google Scholar]
- Bedi, G.; Tonzibo, Z.F.; Nguessan, T.Y. Composition chimique des huiles essentielles de Chromolaena odorata L. King Robinson d’Abidjan-Cote d’Ivoire. J. Soc. Ouest-Afr. Chim. 2001, 11, 29–37. [Google Scholar]
- Pisutthanan, N.; Liawruangrath, B.; Liawruangrath, S. Constituents of the essential oil from aerial parts of Chromolaena odorata from Thailand. Nat. Prod. Res. 2006, 20, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Dare, E.O.; Inyang, D.S.; Onwuka, J.C. 2-Imino-(3,4-dimethoxybenzyl) ethanesulfonic acid Schiff base anchored silver nanocomplex mediated by sugarcane juice and their antibacterial activities. J. Appl. Res. Technol. 2016, 14, 38–46. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Katata-Seru, L. Biosynthesis, characterization, and antimicrobial effect of silver nanoparticles using Lavandula x intermedia. Res. Chem. Intermed. 2016. [Google Scholar] [CrossRef]
- Rahuman, A.A.; Gopalakrishnan, G.; Ghouse, B.S.; Arumugam, S.; Himalayan, B. Effect of Feronia limonia on mosquito larvae. Fitoterapia 2000, 71, 553–555. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization Document WHO, CDS/ WHO-PES/GCDPP/13; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- McCracken, W.A.; Cowsan, R.A. Clinical and Oral Microbiology; Hemispher Publishing Corporation: New York, NY, USA, 1983. [Google Scholar]
- Khan, A.U.; Wei, Y.; Ahmad, A.; Khan, Z.U.; Tahir, K.; Khan, S.U.; Muhammad, N.; Khan, F.U.; Yuan, Q. Enzymatic browning reduction in white cabbage, potent antibacterial and antioxidant activities of biogenic silver nanoparticles. J. Mol. Liqs. 2016, 215, 39–46. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Gnanasekar, S.; Seetharaman, P.; Keppanan, R.; Arockiaswamy, W.; Sivaperumal, S. Formulation of Carica papaya latex-functionalized silver nanoparticles for its improved antibacterial and anticancer applications. J. Mol. Liq. 2016, 219, 232–238. [Google Scholar] [CrossRef]
- Belkys, F.; Isabe, L.I.; Josep, G. Evaluation of Disk Diffusion Method for Determining Eberconazole Susceptibility of Dermatophytes and Influence of Culture Medium. Antimicrob. Agents Chemother. 2005, 49, 2116–2118. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard M27-A2; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002. [Google Scholar]
- Simon, H.J.; Yin, E.J. Microbioassay of antimicrobial agents. Appl. Microbiol. 1970, 19, 573–579. [Google Scholar] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Shultz, D.A.; Shultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface Plasmon Spectroscopy of Nanosized Metal Particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Gole, A.; Dash, C.; Ramakrishnaan, V.; Sainkar, S.R.; Mandal, A.B.; Rao, M.; Sastry, M. Pepsin–Gold Colloid Conjugates: Preparation, Characterization, and Enzymatic Activity. Langmuir 2001, 17, 1674–1679. [Google Scholar] [CrossRef]
- Armani, M.A.; Abu-Taleb, A.; Remalli, N.; Abdullah, M.; Srikanth, V.V.S.S.; Labhasetwar, N.K. Dragon’s blood-aided synthesis of Ag/Ag2O core/shell nanostructures and Ag/Ag2O decked multilayered graphene for efficient As(III) uptake from water and antibacterial activity. RSC Adv. 2016, 6, 44145–44153. [Google Scholar] [CrossRef]
- Murugan, K.; Benelli, G.; Ayyappan, S.; Dinesh, D.; Panneerselvam, C.; Nicoletti, M.; Hwang, J.S.; Mahesh, K.P.; Subramaniam, J.; Suresh, U. Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasitol. Res. 2015, 114, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Mehlhorn, H. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol. Res. 2006, 99, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Subashini, K.; Jeyasankar, A.; Ramesh, N.; Sivakami, R. Larvicidal activity of silver nanoparticle synthesized by the leaf extracts of Azadirachta indica against Culex quinquefasciatus (Say) (Diptera: Culicidae). Int. J. Zool. Stud. 2016, 1, 7–11. [Google Scholar]
- Satyavani, K.; Ramanathan, T.; Gurudeeban, S. Green synthesis of silver nanoparticles by using stem derived callus extract of Bitter apple (Citrullus colocynthis). Dig. J. Nanomater. Biostruct. 2011, 6, 1019–1024. [Google Scholar]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interf. Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Sujitha, V.; Murugan, K.; Paulpandi, M. Green synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol. Res. 2015, 114, 3315–3325. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



 silver,
silver,  silver oxide).
silver oxide).


| Sample | Larvae Instar | Conc. (ppm) | Exposure Time (h) | %Mortality ± Standard Error | LC50 (ppm) | LC90 (ppm) | χ2 (d = 4) (n.s.) |
|---|---|---|---|---|---|---|---|
| EO-AgNPs | III | 50 | 12 | 34.67 ± 0.33 | 148.5 | 420.9 | 0.017 |
| 100 | 40.00 ± 0.58 | ||||||
| 150 | 46.67 ± 0.33 | ||||||
| 200 | 58.67 ± 0.33 | ||||||
| 250 | 70.67 ± 0.33 | ||||||
| 50 | 24 | 42.67 ± 0.33 | 95.9 | 337.5 | 0.983 | ||
| 100 | 49.33 ± 0.33 | ||||||
| 150 | 60.00 ± 1.16 | ||||||
| 200 | 68.00 ± 1.00 | ||||||
| 250 | 81.33 ± 0.88 | ||||||
| IV | 50 | 12 | 25.32 ± 0.67 | 217.1 | 483.8 | 0.585 | |
| 100 | 25.33 ± 0.33 | ||||||
| 150 | 33.33 ± 0.67 | ||||||
| 200 | 46.67 ± 0.67 | ||||||
| 250 | 60.00 ± 1.16 | ||||||
| 50 | 24 | 33.33 ± 0.33 | 166.4 | 438.7 | 1.111 | ||
| 100 | 34.67 ± 0.88 | ||||||
| 150 | 45.33 ± 0.88 | ||||||
| 200 | 49.33 ± 0.33 | ||||||
| 250 | 70.67 ± 0.88 | ||||||
| Plant Extract (EO) | III | 50 | 12 | 6.67 ± 0.33 | 461.6 | 807.6 | 0.013 |
| 100 | 9.33 ± 0.33 | ||||||
| 150 | 12.00 ± 0.00 | ||||||
| 200 | 14.67 ± 0.33 | ||||||
| 250 | 24.00 ± 0.58 | ||||||
| 50 | 24 | 8.00 ± 0.58 | 396.8 | 716.8 | 0.781 | ||
| 100 | 13.33 ± 0.33 | ||||||
| 150 | 16.00 ± 0.00 | ||||||
| 200 | 17.33 ± 0.33 | ||||||
| 250 | 30.67 ± 0.33 | ||||||
| IV | 50 | 12 | 5.32 ± 0.33 | 553.4 | 953.4 | 0.612 | |
| 100 | 8.00 ± 0.00 | ||||||
| 150 | 9.32 ± 0.33 | ||||||
| 200 | 12.00 ± 0.00 | ||||||
| 250 | 17.33 ± 0.67 | ||||||
| 50 | 24 | 9.33 ± 0.33 | 448.3 | 803.9 | 5.162 | ||
| 100 | 9.33 ± 0.67 | ||||||
| 150 | 12.00 ± 0.00 | ||||||
| 200 | 17.33 ± 0.67 | ||||||
| 250 | 26.67 ± 0.33 |
| Microbial Strains | MIC |
|---|---|
| Staphylococcus aureus | 75 μg/mL |
| Bacillus subtilis | 75 μg/mL |
| Salmonella typhi | 40 μg/mL |
| Escherichia coli | 25 μg/mL |
| Candida albicans | 100 μg/mL |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Sonde, C.U.; Ehiri, R.C. Green Synthesis of Ag/Ag2O Nanoparticles Using Aqueous Leaf Extract of Eupatorium odoratum and Its Antimicrobial and Mosquito Larvicidal Activities. Molecules 2017, 22, 674. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050674
Elemike EE, Onwudiwe DC, Ekennia AC, Sonde CU, Ehiri RC. Green Synthesis of Ag/Ag2O Nanoparticles Using Aqueous Leaf Extract of Eupatorium odoratum and Its Antimicrobial and Mosquito Larvicidal Activities. Molecules. 2017; 22(5):674. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050674
Chicago/Turabian StyleElemike, Elias E., Damian C. Onwudiwe, Anthony C. Ekennia, Christopher U. Sonde, and Richard C. Ehiri. 2017. "Green Synthesis of Ag/Ag2O Nanoparticles Using Aqueous Leaf Extract of Eupatorium odoratum and Its Antimicrobial and Mosquito Larvicidal Activities" Molecules 22, no. 5: 674. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050674