New Glycosides from the Fruits of Nicandra physaloides
Abstract
:1. Introduction
2. Results
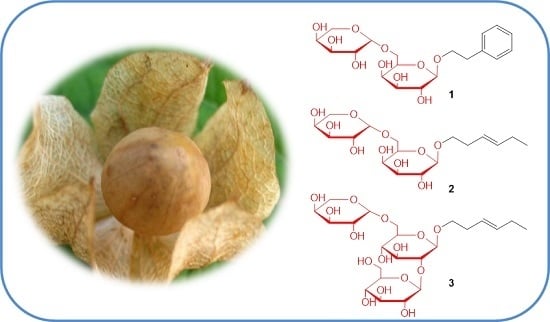
2.1. Structure Elucidation
2.2. Anti-Inflammatory Activity
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extration and Isolation
3.4. Acid Hydrolysis and GC Analysis
3.5. Anti-Inflammatory Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HR-ESI-MS | High-resolution electrospray ionization mass spectrometry |
| NMR | Nuclear magnetic resonance |
| DEPT | Distortionless Enhancement by Polarization Transfer |
| HMBC | Heteronuclear multiple bond correlation |
| 1H-1H COSY | Correlation spectroscopy |
| HSQC | Heteronuclear multiple quantum coherence |
| GC | Gas chromatography |
| tR | Retention time |
| NMMA | NG-monomethyl Arginine |
| UV | Ultraviolet |
| TMS | Tetramethylsilane |
| HPLC | High performance liquid chromatography |
| ODS | Octadecylsilyl |
| ELISA | Enzyme-linked immunosorbent assay |
| DMEM | Dulbecco’s modified eagle medium |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| LPS | Lipopolysaccharides |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate buffer saline |
| EtOH | Ethyl alcohol |
| TLC | Thin Layer Chromatography |
| MeOH | Methanol |
| HMDS-TMCS | Hexamethyldisi lazane-trimethylchlorosilane |
References
- Nanjing University of Chinese Medicine. Dictionary of Medicinal Plants; Shanghai Science and Technology Publishing House: Shanghai, China, 2006. [Google Scholar]
- Xie, Z.W.; Deng, J.L.; Li, T.C. Quanguo Zhongcaoyao Huibian; People’s Medical Publishing House: Beijing, China, 1987. [Google Scholar]
- Tu, G.Y.; Wang, Z.W.; Ou, H. Study on Rheological Properties of Mixed Xanthan Gum and Gum in Seed of Nicandra physaloides (L.) Gaertn. Nat. Prod. Res. Dev. 2010, 22, 285–288. [Google Scholar]
- Fan, Z.Y.; Zhang, L.Q.; Li, L.H. Effect of different medium on inducement and differentiation of Nicandra physaloides. J. Ankang Univ. 2008, 5, 83–84. [Google Scholar]
- Gunasekera, S.P.; Cordell, G.A.; Farnsworth, N.R. Plant anticancer agents XX.* constituents of Nicandra physalodes. Planta Med. 1981, 43, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Meera, R.; Muthumani, P. Evaluation of alcoholic and aqueous extracts of Nicandra physalodes leaves for diuretic activity. Int. J. Pharm. Biol. Arch. 2010, 1, 331–334. [Google Scholar]
- Begley, M.J.; Crombie, L.; Ham, P.J. Constitution of four novel methyl steroid relatives (ring-D aromatic) from the insect repellent plant Nicandra physaloides; X-ray analysis of Nic-10. J. Chem. Soc. Chem. Commun. 1972, 22, 1250–1251. [Google Scholar] [CrossRef]
- Zhao, G.T. Study on the chemical constituents and bioactivities of the seeds of Momordica charantia and niacin. Kunming Univ. Sci. Technol. 2015. [Google Scholar]
- Kawahara, E.; Fujii, M.; Kato, K. Chemoenzymatic synthesis of naturally occurring benzyl 6-O-glycosyl-β-d-glucopyranosides. Chem. Pharm. Bull. 2005, 53, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Xiao, B.; Fu, H.Z.; Xu, Y. Phytochemical study of Asparagus officinalis. Mod. Chin. Med. 2016, 18, 1571–1573. [Google Scholar]
- Umehara, K.; Hattori, I.; Miyase, T. Studies on the Constituents of Leaves of Citrus unshiu MARCOV. Chem. Pharm. Bull. 1988, 36, 5004–5008. [Google Scholar] [CrossRef]
- Wang, F.Q.; Han, S.S.; Hu, S.; Xue, Y.B.; Wang, J.P.; Xu, H.F.; Chen, L.; Zhang, G.; Zhang, Y.H. Two new secondary metabolites from Xylaria sp. CFCC 87468. Molecules 2014, 19, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, M.A.D.; Galotta, A.L.Q.A. Polar constituents of Euterpe precatoria roots and their plant growth activity. Chem. Nat. Compd. 2009, 45, 588–589. [Google Scholar] [CrossRef]
- Wang, J.; Xia, F.; Sun, J.; Jiang, Y.; Tu, P.F. Chemical constituents of Aquilaria sinensis (Lour.) Gilg. China J. Chin. Mater. Med. 2013, 38, 3299–3303. [Google Scholar]
- Wang, L.B.; Wang, C.; Sun, S.C.; Xu, B.; Wu, L.J. Chemical constituents in the lipid-lowering fraction of flos Helichrysum arenarium (III). Chin. J. Med. Chem. 2012, 22, 220–222. [Google Scholar]
- Endo, K.; Hikino, H. Structures of rengyol, rengyoxide, and rengyolone, new cyclohexylethane derivatives from Forsythia suspensa fruits. Can. J. Chem. 1984, 62, 2011–2014. [Google Scholar] [CrossRef]
- Masateru, O.; Shiono, Y.; Tanaka, T.; Masuoka, C.; Shin, Y. Three new aromatic glycosides from the ripe fruit of cherry tomato. J. Nat. Med. 2010, 64, 500–505. [Google Scholar]
- Wu, T.; Li, Y.; Kong, D.Y.; Li, H.T. Chemical constituents of Helicia nilagirica Beed. Chin. Pharm. J. 2010, 45, 1224–1227. [Google Scholar]
- Wu, Z.J.; Ouyang, M.A.; Wang, S.B. Two new phenolic water-soluble constituents from branch bark of Davidia involucrata. Nat. Prod. Res. 2008, 22, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.M.; Li, S.L.; Zhao, Q.; Hao, X.J. Butyl acetals from medicinal plant, Winchia calophylla (Apocynaceae). Acta Bot. Yunnanica 2007, 29, 708–712. [Google Scholar]
- Nakamura, S.; Zhang, Y.; Matsuda, H.; Ninomiya, K.; Muraoka, O.; Yoshikawa, M. Chemical Structures and Hepatoprotective Effects of Constituents from the Leaves of Salacia chinensis. Chem. Pharm. Bull. 2011, 59, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Yuda, M.; Tanaka, O.; Saruwatari, Y.; Fuwa, T.; Jia, M.R.; Ling, Y.K.; Pu, X.F. Chemical studies on the Chinese traditional medicine, dangshen. I. Isolation of (Z)-3- and (E)-2-hexenyl β-d-glucosides. Chem. Pharm. Bull. 1988, 36, 2689–2690. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Li, Z.L.; Wang, X.; Yang, Y.P.; Peng, W.B.; Liu, K.C.; Li, X.L. Chemical constituents from roots of Campanumoea javanica and their antiangiogeneic activities. Chin. Tradit. Herb Drugs 2015, 4, 470–475. [Google Scholar]
- Wang, S.F.; Ghisalberti, E.L.; Smith, J.R. Bioactive isoflavonols and other components from Trifolium subterraneum. J. Nat. Prod. 1998, 61, 508–510. [Google Scholar] [CrossRef]
- Kijima, H.; Ide, T.; Otsuka, H.; Ogimi, C.; Takushi, A.; Takeda, Y. Water-soluble phenolic glycosides from leaves of Alangium premnifolium. Phytochemistry 1997, 44, 1551–1557. [Google Scholar] [CrossRef]
- Ding, Y.; Liang, C.; Choi, E.M.; Ra, J.C.; Kim, Y.H. Chemical constituents from Artemisia iwayomogi increase the function of osteoblastic MC3T3-E1 cells. Nat. Prod. Sci. 2009, 15, 192–197. [Google Scholar]
- Takashi, T.; Osamu, T.; Lin, W.Z.; Zhou, J.; Hiroyuki, A. Sweet and bitter glycosides of the Chinese plant drug, bai-yun-shen (roots of Salvia digitaloides). Chem. Pharm. Bull. 1983, 31, 780–783. [Google Scholar]
- Kellum, J.A.; Song, M.; Li, J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R686–R692. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Israf, D.A.; Lajis, N.H. Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. Eur. J. Pharmacol. 2006, 538, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.B.; Meyer, R.S.; Whitaker, B.D.; Litt, A.; Kennelly, E.J. Antioxidant glucosylated caffeoylquinic acid derivatives in the invasive tropica soda apple, Solanum viarum. J. Nat. Prod. 2012, 75, 2246–2250. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Guo, R.; Li, T. Five withanolides from the leaves of Datura metel L. and their inhibitory effects on nitric oxide production. Molecules 2014, 19, 4548–4559. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Yang, B.Y.; Guo, R.; Li, T.; Wu, J.J.; Zhang, J.; Liu, Y.; Wang, Q.H.; Kuang, H.X. New anti-inflammatory withanolides from the leaves of Datura metel L. Steroids 2014, 87, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Julião, L.D.S.; Piccinelli, A.L.; Marzocco, S. Phenylethanoid Glycosides from Lantana fucata with in Vitro Anti-inflammatory Activity. J. Nat. Prod. 2009, 72, 1424. [Google Scholar]
- Chen, C.H.; Song, T.Y.; Liang, Y.C. Acteoside and 6-O-acetylacteoside downregulate cell adhesion molecules induced by il-1β through inhibition of ERK and JNK in human vascular endothelial cells. J. Agric. Food Chem. 2009, 57, 8852–8859. [Google Scholar] [CrossRef] [PubMed]
- Ai-Zhi, W.U.; Lin, C.Z. Progress in Structure-activity Relationship of Phenylethanoid Glycosides. Nat. Prod. Res. Dev. 2013, 1, 862–865. [Google Scholar]
- Zheng, X.K.; Liu, Y.Y.; Feng, W.S. Development in research of natural phenylethanoid glycosides. Chin. J. New Drugs 2011, 20, 230–234. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH mult (J, Hz) | δC | δH mult (J, Hz) | δC | δH mult (J, Hz) | |
| 1 | 140.2 | 14.6 | 0.96 (3H, t, 7.6) | 14.7 | 0.97 (3H, t, 7.6) | |
| 2 | 129.4 | 7.24 overlap | 21.6 | 2.07 (2H, m) | 21.6 | 2.08 (2H, m) |
| 3 | 130.0 | 7.24 overlap | 134.5 | 5.42 (m) | 134.5 | 5.42 (m) |
| 4 | 127.2 | 7.16 (m) | 125.9 | 5.42 (m) | 126.0 | 5.42 (m) |
| 5 | 130.0 | 7.24 overlap | 28.8 | 2.37 (2H, m) | 28.8 | 2.38 (2H, m) |
| 6 | 129.4 | 7.24 overlap | 70.6 | 3.52 overlap | 70.6 | 3.54 overlap |
| 3.84 overlap | 3.85 overlap | |||||
| 7 | 37.3 | 2.92 (t, 7.4) | ||||
| 8 | 71.8 | 3.74 overlap | ||||
| 4.07 overlap | ||||||
| 1′ | 102.0 | 4.65 (d, 7.9) | 102.0 | 4.61 (d, 8.0) | 105.0 | 4.59 (d, 7.8) |
| 2′ | 72.3 | 3.34 overlap | 72.3 | 3.51 (m) | 83.0 | 3.20–3.80 (m) |
| 3′ | 72.8 | 4.04 (m) | 72.8 | 4.05 (m) | 78.2 | 3.20–3.80 (m) |
| 4′ | 68.8 | 3.55 (m) | 68.8 | 3.55 (m) | 71.4 | 3.20–3.80 (m) |
| 5′ | 74.5 | 3.83 (m) | 74.4 | 3.82 (m) | 77.7 | 3.20–3.80 (m) |
| 6′ | 69.8 | 4.09 overlap | 69.8 | 4.06 (dd, 11.2, 2.0) | 69.5 | 4.09 (dd, 11.2, 1.9) |
| 3.70 overlap | 3.72 (dd, 11.2, 5.0) | 3.70 (dd, 11.4, 5.1) | ||||
| 1″ | 105.2 | 4.29 (d, 6.7) | 105.2 | 4.30 (d, 6.7) | 105.1 | 4.30 (d, 6.7) |
| 2″ | 72.4 | 3.57 (m) | 72.4 | 3.59 (m) | 72.4 | 3.20–3.80 (m) |
| 3″ | 74.2 | 3.47 (m) | 74.2 | 3.50 (m) | 74.2 | 3.20–3.80 (m) |
| 4″ | 69.5 | 3.78 (m) | 69.5 | 3.80 (m) | 69.5 | 3.20–3.80 (m) |
| 5″ | 66.7 | 3.50 (dd,12.5,3.2) | 66.7 | 3.52 overlap | 66.7 | 3.51 overlap |
| 3.85 (dd,12.5,2.0) | 3.87 overlap | 3.87 overlap | ||||
| 1″′ | 103.0 | 4.43 (d, 7.6) | ||||
| 2″′ | 76.0 | 3.20–3.80 (m) | ||||
| 3″′ | 77.7 | 3.20–3.80 (m) | ||||
| 4″′ | 71.4 | 3.20–3.80 (m) | ||||
| 5″′ | 76.7 | 3.20–3.80 (m) | ||||
| 6″′ | 62.7 | 3.52 overlap | ||||
| 3.87 overlap | ||||||
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| NMMA b | 19.6 ± 2.4 | ||
| Compound 1 | 31.1 ± 3.5 | Compound 10 | >50 |
| Compound 2 | 32.9 ± 5.6 | Compound 11 | 41.7 ± 7.6 |
| Compound 3 | 41.2 ± 4.1 | Compound 12 | 29.8 ± 5.7 |
| Compound 4 | 30.2 ± 4.7 | Compound 13 | 36.6 ± 3.9 |
| Compound 5 | 26.9 ± 5.1 | Compound 14 | >50 |
| Compound 6 | 37.5 ± 4.7 | Compound 15 | 25.1 ± 4.4 |
| Compound 7 | 41.2 ± 6.6 | Compound 16 | 38.9 ± 5.9 |
| Compound 8 | >50 | Compound 17 | 33.4 ± 2.7 |
| Compound 9 | 34.8 ± 6.3 | Compound 18 | 31.4 ± 4.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, H.-B.; Xu, Z.-P.; Cheng, Y.-G.; Lv, S.-W.; Yang, B.-Y.; Guo, H.-W.; Kuang, H.-X. New Glycosides from the Fruits of Nicandra physaloides. Molecules 2017, 22, 828. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050828
Liu Y, Jiang H-B, Xu Z-P, Cheng Y-G, Lv S-W, Yang B-Y, Guo H-W, Kuang H-X. New Glycosides from the Fruits of Nicandra physaloides. Molecules. 2017; 22(5):828. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050828
Chicago/Turabian StyleLiu, Yan, Hai-Bing Jiang, Zhen-Peng Xu, Yan-Gang Cheng, Shao-Wa Lv, Bing-You Yang, Hong-Wei Guo, and Hai-Xue Kuang. 2017. "New Glycosides from the Fruits of Nicandra physaloides" Molecules 22, no. 5: 828. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050828