Electrophilic Trifluoromethylselenolation of Boronic Acids
Abstract
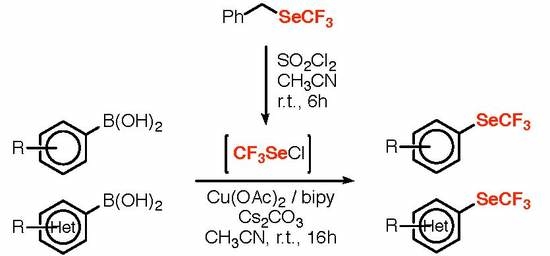
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Benzyl Trifluoromethyl Selenide (1)
3.2. Typical Procedure






4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smart, B.E. Fluorine substituent effects (on bioactivity). J. Fluor. Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Becker, A. Inventory of Industrial Fluoro-Biochemicals; Eyrolles: Paris, France, 1996. [Google Scholar]
- Fujiwara, T.; O’Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluor. Chem. 2014, 167, 16–29. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Hird, M. Fluorinated liquid crystals—Properties and applications. Chem. Soc. Rev. 2007, 36, 2070–2095. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, M.; Ciriminna, R. New fluorinated functional materials. J. Mater. Chem. 2005, 15, 4981–4991. [Google Scholar] [CrossRef]
- Theodoridis, G. Chapter 4 Fluorine-Containing Agrochemicals: An Overview of Recent Developments. In Advances in Fluorine Science; Tressaud, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 121–175. [Google Scholar]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag. Sci. 2010, 66, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.; Row, T.N.G. Role of organic fluorine in crystal engineering. CrystEngComm 2011, 13, 2175–2186. [Google Scholar] [CrossRef]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Toulgoat, F.; Alazet, S.; Billard, T. Direct Trifluoromethylthiolation Reactions: The “Renaissance” of an Old Concept. Eur. J. Org. Chem. 2014, 2415–2428. [Google Scholar] [CrossRef]
- Xu, X.-H.; Matsuzaki, K.; Shibata, N. Synthetic Methods for Compounds Having CF3–S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions. Chem. Rev. 2015, 115, 731–764. [Google Scholar] [CrossRef]
- Tlili, A.; Toulgoat, F.; Billard, T. Synthetic Approaches to Trifluoromethoxy-Substituted Compounds. Angew. Chem. Int. Ed. 2016, 55, 11726–11735. [Google Scholar] [CrossRef]
- Leroux, F.R.; Manteau, B.; Vors, J.-P.; Pazenok, S. Trifluoromethyl ethers—Synthesis and properties of an unusual substituent. Beilstein J. Org. Chem. 2008, 4, 13. [Google Scholar] [CrossRef]
- Ben-David, I.; Rechavi, D.; Mishani, E.; Rozen, S. A novel synthesis of trifluoromethyl ethers via xanthates, utilizing BrF3. J. Fluor. Chem. 1999, 97, 75–78. [Google Scholar] [CrossRef]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Abdulah, R.; Miyazaki, K.; Nakazawa, M.; Koyama, H. Chemical forms of selenium for cancer prevention. J. Trace Elem. Med. Biol. 2005, 19, 141–150. [Google Scholar] [CrossRef]
- Bodnar, M.; Konieczka, P.; Namiesnik, J. The Properties, Functions, and Use of Selenium Compounds in Living Organisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2012, 30, 225–252. [Google Scholar] [CrossRef]
- Brown, K.M.; Arhur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef]
- Combs, G.F.; Gray, W.P. Chemopreventive agents: Selenium. Pharmacol. Therap. 1998, 79, 179–192. [Google Scholar] [CrossRef]
- Holben, D.H.; Smith, A.M. The diverse role of selenium within selenoproteins: A review. J. Am. Diet. Assoc. 1999, 99, 836–843. [Google Scholar] [CrossRef]
- Kyriakopoulos, A.; Behne, D. Selenium-containing proteins in mammals and other forms of life. Rev. Physiol. Biochem. Pharmacol. 2002, 145, 1–46. [Google Scholar]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet. 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Romashov, L.V.; Ananikov, V.P. Self-Assembled Selenium Monolayers: From Nanotechnology to Materials Science and Adaptive Catalysis. Chem. Eur. J. 2013, 19, 17640–17660. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Schneider, A.; Abbas, M.; Brandt, W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol. Chem. 2007, 388, 997–1006. [Google Scholar] [CrossRef]
- Angeli, A.; Tanini, D.; Viglianisi, C.; Panzella, L.; Capperucci, A.; Menichetti, S.; Supuran, C.T. Evaluation of selenide, diselenide and selenoheterocycle derivatives as carbonic anhydrase I, II, IV, VII and IX inhibitors. Bioorg. Med. Chem. 2017, 25, 2518–2523. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Kaczor, K.B.; Wojtowicz, A.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Ścianowski, J. New glutathione peroxidase mimetics—Insights into antioxidant and cytotoxic activity. Bioorg. Med. Chem. 2017, 25, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Lynch, E.D.; Gu, R.; Pierce, C.; Kil, J. Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear. Res. 2005, 201, 81–89. [Google Scholar] [CrossRef]
- Singh, N.; Sharpley, A.L.; Emir, U.E.; Masaki, C.; Herzallah, M.M.; Gluck, M.A.; Sharp, T.; Harmer, C.J.; Vasudevan, S.R.; Cowen, P.J.; et al. Effect of the Putative Lithium Mimetic Ebselen on Brain Myo-Inositol, Sleep, and Emotional Processing in Humans. Neuropsychopharmacology 2016, 41, 1768–1778. [Google Scholar] [CrossRef]
- Thangamani, S.; Younis, W.; Seleem, M.N. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci. Rep. 2015, 5, 11596. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Halliday, A.C.; Thomas, J.M.; Kuznetsova, O.V.; Baldwin, R.; Woon, E.C.Y.; Aley, P.K.; Antoniadou, I.; Sharp, T.; Vasudevan, S.R.; et al. A safe lithium mimetic for bipolar disorder. Nat. Commun. 2013, 4, 1332. [Google Scholar] [CrossRef]
- Glenadel, Q.; Ismalaj, E.; Billard, T. Electrophilic Trifluoromethyl- and Fluoroalkylselenolation of Organometallic Reagents. Eur. J. Org. Chem. 2017, 2017, 530–533. [Google Scholar] [CrossRef]
- Billard, T.; Langlois, B.R. A new simple access to trifluoromethyl thioethers or selenoethers from trifluoromethyl trimethylsilane and disulfides or diselenides. Tetrahedron Lett. 1996, 37, 6865–6868. [Google Scholar] [CrossRef]
- Billard, T.; Large, S.; Langlois, B.R. Preparation of trifluoromethyl sulfides or selenides from trifluoromethyl trimethylsilane and thiocyanates or selenocyanates. Tetrahedron Lett. 1997, 38, 65–68. [Google Scholar] [CrossRef]
- Billard, T.; Langlois, B.R.; Large, S. Synthesis of Trifluoromethyl Selenides. Phosphorus Sulfur Silicon Relat. Elem. 1998, 136, 521–524. [Google Scholar] [CrossRef]
- Billard, T.; Roques, N.; Langlois, B.R. Synthetic Uses of Thio- and Selenoesters of Trifluoromethylated Acids. 1. Preparation of Trifluoromethyl Sulfides and Selenides. J. Org. Chem. 1999, 64, 3813–3820. [Google Scholar] [CrossRef]
- Large, S.; Roques, N.; Langlois, B.R. Nucleophilic Trifluoromethylation of Carbonyl Compounds and Disulfides with Trifluoromethane and Silicon-Containing Bases. J. Org. Chem. 2000, 65, 8848–8856. [Google Scholar] [CrossRef]
- Blond, G.; Billard, T.; Langlois, B.R. New stable reagents for the nucleophilic trifluoromethylation. Part 4: Trifluoromethylation of disulfides and diselenides with hemiaminals of trifluoroacetaldehyde. Tetrahedron Lett. 2001, 42, 2473–2475. [Google Scholar] [CrossRef]
- Magnier, E.; Vit, E.; Wakselman, C. A Convenient Access to Perfluoroalkyl Selenoethers and Selenyl Chlorides. Synlett 2001, 1260–1262. [Google Scholar] [CrossRef]
- Pooput, C.; Medebielle, M.; Dolbier, W.R. A New and Efficient Method for the Synthesis of Trifluoromethylthio- and Selenoethers. Org. Lett. 2004, 6, 301–303. [Google Scholar] [CrossRef]
- Pooput, C.; Dolbier, W.R.; Médebielle, M. Nucleophilic Perfluoroalkylation of Aldehydes, Ketones, Imines, Disulfides, and Diselenides. J. Org. Chem. 2006, 71, 3564–3568. [Google Scholar] [CrossRef]
- Cherkupally, P.; Beier, P. Alkoxide-induced nucleophilic trifluoromethylation using diethyl trifluoromethylphosphonate. Tetrahedron Lett. 2010, 51, 252–255. [Google Scholar] [CrossRef]
- Potash, S.; Rozen, S. General Synthesis of Trifluoromethyl Selenides Utilizing Selenocyanates and Fluoroform. J. Org. Chem. 2014, 79, 11205–11208. [Google Scholar] [CrossRef]
- Ma, J.-J.; Yi, W.-B.; Lu, G.-P.; Cai, C. Trifluoromethylation of thiophenols and thiols with sodium trifluoromethanesulfinate and iodine pentoxide. Catal. Sci. Technol. 2016, 6, 417–421. [Google Scholar] [CrossRef]
- Nikolaienko, P.; Rueping, M. Trifluoromethylselenolation of Aryldiazonium Salts: A Mild and Convenient Copper-Catalyzed Procedure for the Introduction of the SeCF3 Group. Chem. Eur. J. 2016, 22, 2620–2623. [Google Scholar] [CrossRef]
- Kondratenko, N.V.; Kolomeytsev, A.A.; Popov, V.I.; Yagupolskii, L.M. Synthesis and Reactions of Trifluoromethylthio(seleno)- and Pentafluorophenylthio(seleno)-copper. Synthesis 1985, 667–669. [Google Scholar] [CrossRef]
- Zhu, P.; He, X.; Chen, X.; You, Y.; Yuan, Y.; Weng, Z. Copper-mediated synthesis of α-trifluoromethylthio- and seleno-α,β-unsaturated carbonyl compounds. Tetrahedron 2014, 70, 672–677. [Google Scholar] [CrossRef]
- Rong, M.; Huang, R.; You, Y.; Weng, Z. Synthesis of propargylic and allylic trifluoromethyl selenoethers by copper-mediated trifluoromethylselenolation of propargylic chlorides and allylic bromides. Tetrahedron 2014, 70, 8872–8878. [Google Scholar] [CrossRef]
- Chen, C.; Ouyang, L.; Lin, Q.; Liu, Y.; Hou, C.; Yuan, Y.; Weng, Z. Synthesis of CuI Trifluoromethylselenates for Trifluoromethylselenolation of Aryl and Alkyl Halides. Chem. Eur. J. 2014, 20, 657–661. [Google Scholar] [CrossRef]
- Chen, C.; Hou, C.; Wang, Y.; Hor, T.S.A.; Weng, Z. Copper-Catalyzed Trifluoromethylselenolation of Aryl and Alkyl Halides: The Silver Effect in Transmetalation. Org. Lett. 2014, 16, 524–527. [Google Scholar] [CrossRef]
- Wu, C.; Huang, Y.; Chen, Z.; Weng, Z. Synthesis of vinyl trifluoromethyl selenoethers. Tetrahedron Lett. 2015, 56, 3838–3841. [Google Scholar] [CrossRef]
- Wang, Y.; You, Y.; Weng, Z. Alkynyl trifluoromethyl selenide synthesis via oxidative trifluoromethylselenolation of terminal alkynes. Org. Chem. Front. 2015, 2, 574–577. [Google Scholar] [CrossRef]
- Lefebvre, Q.; Pluta, R.; Rueping, M. Copper catalyzed oxidative coupling reactions for trifluoromethylselenolations—Synthesis of R-SeCF3 compounds using air stable tetramethylammonium trifluoromethylselenate. Chem. Commun. 2015, 51, 4394–4397. [Google Scholar] [CrossRef]
- Hou, C.; Lin, X.; Huang, Y.; Chen, Z.; Weng, Z. Synthesis of β-Trifluoromethylthio- and β-Trifluoromethylseleno-α,β-unsaturated Ketones through Copper-Mediated Trifluoromethylthio(seleno)lation. Synthesis 2015, 47, 969–975. [Google Scholar] [CrossRef]
- Aufiero, M.; Sperger, T.; Tsang, A.S.K.; Schoenebeck, F. Highly Efficient C-SeCF3 Coupling of Aryl Iodides Enabled by an Air-Stable Dinuclear PdI Catalyst. Angew. Chem. Int. Ed. 2015, 54, 10322–10326. [Google Scholar] [CrossRef]
- Tian, Q.; Weng, Z. A Convenient Process for the Preparation of Heteroaryl Trifluoromethyl Selenoethers. Chin. J. Chem. 2016, 34, 505–510. [Google Scholar] [CrossRef]
- Matheis, C.; Wagner, V.; Goossen, L.J. Sandmeyer-Type Trifluoromethylthiolation and Trifluoromethylselenolation of (Hetero)Aromatic Amines Catalyzed by Copper. Chem. Eur. J. 2016, 22, 79–82. [Google Scholar] [CrossRef]
- Matheis, C.; Krause, T.; Bragoni, V.; Goossen, L.J. Trifluoromethylthiolation and Trifluoromethylselenolation of α-Diazo Esters Catalyzed by Copper. Chem. Eur. J. 2016, 22, 12270–12273. [Google Scholar] [CrossRef]
- Fang, W.-Y.; Dong, T.; Han, J.-B.; Zha, G.-F.; Zhang, C.-P. Expeditious trifluoromethylthiolation and trifluoromethylselenolation of alkynyl(phenyl)iodoniums by [XCF3]− (X = S, Se) anions. Org. Biomol. Chem. 2016, 14, 11502–11509. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Weng, Z. A general method for synthesis of Se-trifluoromethyl esters through Iron-catalyzed trifluoromethylselenolation of acid chlorides. J. Fluor. Chem. 2017, 193, 24–32. [Google Scholar] [CrossRef]
- Dale, J.W.; Emeleus, H.J.; Haszeldine, R.N. Organometallic and organometalloidal fluorine compounds. Part XIV. Trifluoromethyl derivatives of selenium. J. Chem. Soc. 1958, 2939–2945. [Google Scholar] [CrossRef]
- Yarovenko, N.N.; Shemanina, V.N.; Gazieva, G.B. Preparation of hexafluorodimethyl diselenide from salts of trifluoroacetic acid, and some of its properties. Russ. J. Gen. Chem. 1959, 29, 924–927. [Google Scholar]
- Glenadel, Q.; Ismalaj, E.; Billard, T. Benzyltrifluoromethyl (or Fluoroalkyl) Selenide: Reagent for Electrophilic Trifluoromethyl (or Fluoroalkyl) Selenolation. J. Org. Chem. 2016, 81, 8268–8275. [Google Scholar] [CrossRef]
- Glenadel, Q.; Alazet, S.; Tlili, A.; Billard, T. Mild and Soft Catalyzed Trifluoromethylthiolation of Boronic Acids: The Crucial Role of Water. Chem. Eur. J. 2015, 21, 14694–14698. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Entry | [Cu] | Ligand a | Base | Solvent | 3a (%) b |
|---|---|---|---|---|---|
| 1 | CuI (1 eq.) | L1 (2 eq.) | K2CO3 (1 eq.) | CH3CN | 5 |
| 2 | Cu(OAc)2 (1 eq.) | L1 (2 eq.) | K2CO3 (1 eq.) | CH3CN | 23 |
| 3 | Cu(OAc)2 (1 eq.) | L1 (2 eq.) | - | CH3CN | 0 |
| 4 | Cu(OAc)2 (1 eq.) | - | K2CO3 (1 eq.) | CH3CN | 4 |
| 5 | Cu(OAc)2 (1 eq.) | L1 (1 eq.) | K2CO3 (1 eq.) | CH3CN | 40 |
| 6 | Cu(OAc)2 (1 eq.) | L2 (1 eq.) | K2CO3 (1 eq.) | CH3CN | 14 |
| 7 | Cu(OAc)2 (1 eq.) | L3 (1 eq.) | K2CO3 (1 eq.) | CH3CN | 3 |
| 8 | Cu(OAc)2 (1 eq.) | PPh3 (1 eq.) | K2CO3 (1 eq.) | CH3CN | 0 |
| 9 | Cu(OAc)2 (1 eq.) | L4 (1 eq.) | K2CO3 (1 eq.) | CH3CN | 6 |
| 10 | Cu(OAc)2 (1 eq.) | L5 (1 eq.) | K2CO3 (1 eq.) | CH3CN | 3 |
| 11 | Cu(OAc)2 (1 eq.) | L6 (1 eq.) | K2CO3 (1 eq.) | CH3CN | 0 |
| 12 | Cu(OAc)2 (1 eq.) | L7 (1 eq.) | K2CO3 (1 eq.) | CH3CN | 0 |
| 13 | Cu(OAc)2 (1 eq.) | L1 (1 eq.) | Cs2CO3 (1 eq.) | CH3CN | 70 |
| 14 | Cu(OAc)2 (1 eq.) | L1 (1 eq.) | K3PO4 (1 eq.) | CH3CN | 48 |
| 15 | Cu(OAc)2 (1 eq.) | L1 (1 eq.) | CsF (1 eq.) | CH3CN | 6 |
| 16 | Cu(OAc)2 (1 eq.) | L1 (1 eq.) | Et3N (1 eq.) | CH3CN | 0 |
| 17 | Cu(OAc)2 (1 eq.) | L1 (1 eq.) | Pyridine (1 eq.) | CH3CN | 0 |
| 18 c | Cu(OAc)2 (1 eq.) | L1 (1 eq.) | Cs2CO3 (1 eq.) | CH3CN | 37 |
| 19 | Cu(OAc)2 (0.2 eq.) | L1 (0.2 eq.) | Cs2CO3 (1 eq.) | CH3CN | 56 |
| 20 | Cu(OAc)2 (0.2 eq.) | L1 (0.4 eq.) | Cs2CO3 (1 eq.) | CH3CN | 50 |
| 21 c | Cu(OAc)2 (0.2 eq.) | L1 (0.2 eq.) | Cs2CO3 (1 eq.) | CH3CN | - |
| 22 d | Cu(OAc)2 (0.2 eq.) | L1 (0.2 eq.) | Cs2CO3 (1 eq.) | CH3CN | 60 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiazza, C.; Tlili, A.; Billard, T. Electrophilic Trifluoromethylselenolation of Boronic Acids. Molecules 2017, 22, 833. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050833
Ghiazza C, Tlili A, Billard T. Electrophilic Trifluoromethylselenolation of Boronic Acids. Molecules. 2017; 22(5):833. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050833
Chicago/Turabian StyleGhiazza, Clément, Anis Tlili, and Thierry Billard. 2017. "Electrophilic Trifluoromethylselenolation of Boronic Acids" Molecules 22, no. 5: 833. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050833