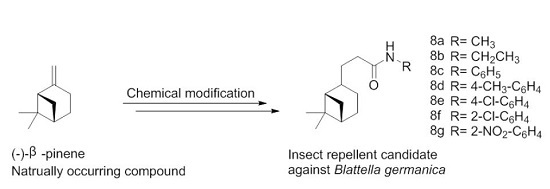
Hydronopylformamides: Modification of the Naturally Occurring Compound (-)-β-Pinene to Produce Insect Repellent Candidates against Blattella germanica
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activity
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. Synthesis of Nopol (2), Hydronopol (3), Hydronopyl Bromide (4), Hydronopyl Formic Acid (6)
3.2.2. Synthesis of Hydronopylformamides (8a–8g) (Taking Compound 8e as An Example)
3.3. Biological Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nasirian, H. Contamination of cockroaches (Insecta: Blattaria) to medically fungi: A systematic review and meta-analysis. J. Med. Mycol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Akinjogunla, O.J.; Odeyemi, A.T.; Udoinyang, E.P. Cockroaches (Periplaneta americana and Blattella germanica): Reservoirs of multi drug resistant (MDR) bacteria in Uyo, Akwa Ibom State. Sci. J. Biol. Sci. 2012, 1, 19–30. [Google Scholar]
- Naqqash, M.N.; Gökçe, A.; Bakhsh, A.; Salim, M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 2016, 115, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.S.; Kaufman, P.E. Natural product pesticides: Their development, delivery and use against insect vectors. Mini-Rev. Org. Chem. 2012, 9, 185–202. [Google Scholar] [CrossRef]
- Omara, S.M.; Al-Ghamdi, K.M.; Mahmoud, M.A.; Sharawi, S.E. Repellency and fumigant toxicity of clove and sesame oils against American cockroach (Periplaneta americana (L.). Afr. J. Biotechnol. 2013, 12, 963–970. [Google Scholar]
- Chao, L.X.; Qiyong, L.; Han, C.; Zhi, L.Q.; Yao, J.S.; Long, L.Z. Evaluation of contact toxicity and repellency of the essential oil of pogostemon cablin leaves and its constituents against Blattella germanica (Blattodae: Blattelidae). J. Med. Entomol. 2015, 52, 86–92. [Google Scholar]
- Zibaee, I.; Khorram, P.B.; Hamoni, M. Evaluation of repellent activity of two essential oils and their mixed formulation against cockroaches (Dictyoptera: Blattidae, Blattellidae) in Iran. J. Entomol. Zool. Stud. 2016, 4, 106–113. [Google Scholar]
- Adzharia, H.N.; Sook, S.Y. Extraction and chemical compositions of Ginger (Zingiber Officinale Roscoe) essential oils as a cockroach repellent. Aust. J. Basic Appl. Sci. 2017, 11, 1–8. [Google Scholar]
- Han, Z.J.; Wang, Z.D.; Jiang, Z.K.; Qian, W.H.; Chen, J.Z.; Zheng, W.Q. Repellent activity evaluation of terpenoids against German cockroaches (in Chinese). Chin. J. Hyg. Insect. Equip. 2012, 18, 290–295. [Google Scholar]
- Katz, T.M.; Miller, J.H.; Hebert, A.A. Insect repellents: Historical perspectives and new developments. J. Am. Acad. Dermatol. 2008, 58, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Osimitz, T.G.; Murphy, J.V.; Fell, L.A.; Page, B. Adverse events associated with the use of insect repellents containing N,N-diethyl-m-toluamide (DEET). Regul. Toxicol. Pharm. 2010, 56, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Corbel, V.; Stankiewicz, M.; Pennetier, C.; Fournier, D.; Stojan, J.; Girard, E.; Dimitrov, M.; Molgó, J.; Hougard, J.M.; Lapied, B. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent DEET. BMC Biol. 2009, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Swale, D.R.; Sun, B.; Tong, F.; Bloomquist, J.R. Neurotoxicity and mode of action of N,N-diethyl-meta-toluamide (DEET). PLoS ONE 2014, 9, e103713. [Google Scholar] [CrossRef] [PubMed]
- McHenry, M.; Lacuesta, G. Severe allergic reaction to diethyltoluamide (DEET) containing insect repellent. Allergy Asthma Clin. Immunol. 2014, 10, a30. [Google Scholar] [CrossRef]
- Costa, V.V.; Bayahia, H.; Kozhevnikova, E.F.; Gusevskaya, E.V.; Kozhevnikov, I.V. Highly active and recyclable metal oxide catalysts for the Prins condensation of biorenewable feedstocks. Chem. Cat Chem. 2014, 6, 2134–2139. [Google Scholar] [CrossRef]
- Zhao, L.H.; Xiao, Z.Q.; Chen, J.Z.; Wang, Z.D.; Fan, G.R.; Chen, S.X. Synthesis and structural analysis of hydronopol and its halides (in Chinese). Chem. Ind. For. Prod. 2012, 32, 39–42. [Google Scholar]
- Liu, Y.; Xiao, Z.Q.; Lu, P.Y.; Chen, J.Z.; Wang, Z.D.; Fan, G.R. Synthesis and structural analysis of hydronopyl formic acid and its esters (in Chinese). Chem. Ind. For. Prod. 2013, 33, 57–61. [Google Scholar]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Yu, M.; Li, X.M.; Wan, T.; Chu, S.S. Repellent activity of eight essential oils of Chinese medicinal herbs to Blattella germanica L. Rec. Nat. Prod. 2011, 5, 176–183. [Google Scholar]
Sample Availability: Samples of the compounds 8a–8g are available from the authors. |


| Compounds | R | Melting Point | Repelling Rate ± Standard Error (%) |
|---|---|---|---|
| 8a | CH3 | 38.0–38.5 | 67.06 ± 3.07 |
| 8b | CH2CH3 | 41.5–42.2 | 38.89 ± 3.61 |
| 8c | C6H5 | 84.7–85.7 | 41.19 ± 6.05 |
| 8d | 4-CH3-C6H4 | 125.1–126.3 | −1.83 ± 5.18 |
| 8e | 4-Cl-C6H4 | 123.2–124.2 | 8.45 ± 7.81 |
| 8f | 2-Cl-C6H4 | 108.8–109.4 | −5.48 ± 9.97 |
| 8g | 2-NO2-C6H4 | 54.3–55.3 | −23.59 ± 6.67 |
| Ethanol | - | - | 0.39 ± 1.18 |
| Acetone | - | - | 0.71 ± 1.42 |
| DEET | - | −33.0 | 54.77 ± 6.61 |
| Compounds | Repelling Rate ± Standard Error (%) | ||
|---|---|---|---|
| 20 mg/mL | 10 mg/mL | 5 mg/mL | |
| 8a | 67.06 ± 3.07 | 50.46 ± 5.07 | 48.26 ± 5.18 |
| DEET | 54.77 ± 6.61 | 34.02 ± 4.41 | 29.56 ± 6.48 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.; Liu, Y.; Si, H.; Xiao, Z.; Fan, G.; Chen, S.; Wang, P.; Wang, Z. Hydronopylformamides: Modification of the Naturally Occurring Compound (-)-β-Pinene to Produce Insect Repellent Candidates against Blattella germanica. Molecules 2017, 22, 1004. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22061004
Liao S, Liu Y, Si H, Xiao Z, Fan G, Chen S, Wang P, Wang Z. Hydronopylformamides: Modification of the Naturally Occurring Compound (-)-β-Pinene to Produce Insect Repellent Candidates against Blattella germanica. Molecules. 2017; 22(6):1004. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22061004
Chicago/Turabian StyleLiao, Shengliang, Yan Liu, Hongyan Si, Zhuanquan Xiao, Guorong Fan, Shangxing Chen, Peng Wang, and Zongde Wang. 2017. "Hydronopylformamides: Modification of the Naturally Occurring Compound (-)-β-Pinene to Produce Insect Repellent Candidates against Blattella germanica" Molecules 22, no. 6: 1004. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22061004