Phase Behaviour and Miscibility Studies of Collagen/Silk Fibroin Macromolecular System in Dilute Solutions and Solid State
Abstract
:1. Introduction
2. Results
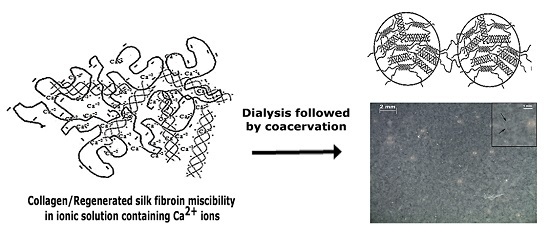
2.1. Miscibility Study of Collagen/Silk Fibroin
2.2. Phase Change after Collagen/Silk Fibroin Dialysis
2.3. Structural Characteristics of Collagen/Silk Fibroin Blend Solutions after Dialysis and Solid Films
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Ternary Phase Diagram and Blend Preparation
4.3. Miscibility and ζ-Potential Analysis
4.4. Light Stereoscopic Magnifier Microscope
4.5. Structure of Collagen/Silk Fibroin Blended Solutions and Films after Dialysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paichit, I.; Atchariya, F.; Anan, O.; Anuphan, S.; Jarupa, V. Effects of the blended fibroin/aloe gel film on wound healing in streptozotocin-induced diabetic rats. Biomed. Mater. 2012, 7, 035008. [Google Scholar]
- Gu, Z.; Xie, H.; Huang, C.; Li, L.; Yu, X. Preparation of chitosan/silk fibroin blending membrane fixed with alginate dialdehyde for wound dressing. Int. J. Biol. Macromol. 2013, 58, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Santos, M.I.; Coutinho, O.P.; Mano, J.F.; Reis, R.L. Physical properties and biocompatibility of chitosan/soy blended membranes. J. Mater. Sci. Mater. Med. 2005, 16, 575–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, E.P.; David, C.W. Energetics of intrachain salt-linkage formation in collagen. Biopolymers 1990, 29, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.P.; David, C.W. Unique side-chain conformation encoding for chirality and azimuthal orientation in the molecular packing of skin collagen. J. Mol. Biol. 1992, 228, 963–969. [Google Scholar] [CrossRef]
- Walker-Taylor, A.; Jones, D.T. Computational methods for predicting protein-protein interactions, In Proteomics and Protein-Protein Interactions: Biology, Chemistry, Bioinformatics, and Drug Design; Waksman, G., Ed.; Springer: Boston, MA, USA, 2005; pp. 89–114. ISBN 978-0-387-24531-7. [Google Scholar]
- Zhang, J. Protein-protein interactions in salt solutions. In Protein-Protein Interactions-Computational and Experimental Tools; Cai, W., Hong, H., Eds.; INTECH Open Access: Rijeka, Croatia, 2012; ISBN 978-953-51-0397-4. [Google Scholar]
- Ross, P.D.; Rekharsky, M.V. Thermodynamics of hydrogen bond and hydrophobic interactions in cyclodextrin complexes. Biophys. J. 1996, 71, 2144–2154. [Google Scholar] [CrossRef]
- Rochdi, A.; Foucat, L.; Renou, J.P. Effect of thermal denaturation on water-collagen interactions: NMR relaxation and differential scanning calorimetry analysis. Biopolymers 1999, 50, 690–696. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silver, F.H.; Landis, W.J. Viscoelasticity, energy Storage and transmission and dissipation by extracellular matrices in vertebrates. In Collagen: Structure and Mechanics; Fratzl, P., Ed.; Springer: Boston, MA, USA, 2008; pp. 133–154. ISBN 978-0-387-73906-9. [Google Scholar]
- Knight, C.G.; Morton, L.F.; Peachey, A.R.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J. Biol. Chem. 2000, 275, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Tzeranis, D.S.; Harley, B.A.; So, P.T.C. Biologically active collagen-based scaffolds: Advances in processing and characterization. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 2123–2139. [Google Scholar] [CrossRef] [PubMed]
- Sah, M.; Pramanik, K. Regenerated silk fibroin from B. mori silk cocoon for tissue engineering applications. Int. J. Environ. Sci. Dev. 2010, 1, 404–408. [Google Scholar] [CrossRef]
- Foo, C.W.P.; Bini, E.; Hensman, J.; Knight, D.P.; Lewis, R.V.; Kaplan, D.L. Role of pH and charge on silk protein assembly in insects and spiders. Appl. Phys. A 2006, 82, 223–233. [Google Scholar] [CrossRef]
- Sashina, E.S.; Bochek, A.M.; Novoselov, N.P.; Kirichenko, D.A. Structure and solubility of natural silk fibroin. Russ. J. Appl. Chem. 2006, 79, 869–876. [Google Scholar] [CrossRef]
- Jin, H.-J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Ochi, A.; Hossain, K. S.; Magoshi, J.; Nemoto, N. Rheology and dynamic light scattering of silk fibroin solution extracted from the middle division of bombyx mori silkworm. Biomacromolecules 2002, 3, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, L.; Cao, X.; Liu, Y. Structure and microporous formation of cellulose/silk fibroin blend membranes: Part II. Effect of post-treatment by alkali. J. Membr. Sci. 2002, 210, 379–387. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, C.; Ma, X.; Chen, C.; Zhu, H. Study on the preparation of collagen-modified silk fibroin films and their properties. Biomed. Mater. 2006, 1, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Chomchalao, P.; Pongcharoen, S.; Sutheerawattananonda, M.; Tiyaboonchai, W. Fibroin and fibroin blended three-dimensional scaffolds for rat chondrocyte culture. Biomed. Eng. Online 2013, 12, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Feng, Q.; Hu, K.; Cui, F. Preparation of three-dimensional fibroin/collagen scaffolds in various pH conditions. J. Mater. Sci. Mater. Med. 2008, 19, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Lv, Q.; Cui, F.Z.; Feng, Q.L.; Kong, X.D.; Wang, H.L.; Hunag, L.Y.; Li, T. Biocompatible fibroin blended films with recombinant human-like collagen for hepatic tissue engineering. J. Bioact. Compat. Polym. 2006, 21, 23–37. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, K.; Feng, Q.; Cui, F. Preparation of insoluble fibroin/collagen films without methanol treatment and the increase of its flexibility and cytocompatibility. J. Appl. Polym. Sci. 2008, 109, 1577–1584. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, K.; Feng, Q.; Cui, F. Fibroin/collagen hybrid hydrogels with crosslinking method: preparation, properties, and cytocompatibility. J. Biomed. Mater. Res. A 2008, 84, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Feng, Q.; Hu, K.; Cui, F. Three-dimensional fibroin/collagen scaffolds derived from aqueous solution and the use for HepG2 culture. Polymer 2005, 46, 12662–12669. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, C.; Ma, X.; Lin, J. Electrospinning of silk fibroin and collagen for vascular tissue engineering. Int. J. Biol. Macromol. 2010, 47, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Krigbaum, W.R.; Wall, F.T. Viscosities of binary polymeric mixtures. J. Polym. Sci. 1950, 5, 505–514. [Google Scholar] [CrossRef]
- García, R.; Melad, O.; Gomez, C.M.; Figueruelo, J.E.; Campos, A. Viscometric study on the compatibility of polymer–polymer mixtures in solution. Eur. Polym. J. 1999, 35, 47–55. [Google Scholar] [CrossRef]
- Wang, Q.; Schlenoff, J.B. The polyelectrolyte complex/coacervate continuum. Macromolecules 2014, 47, 3108–3116. [Google Scholar] [CrossRef]
- van der Gucht, J.; Spruijt, E.; Lemmers, M.; Stuart, M.A.C. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid. Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Läuchli, A.; Polito, V.S. Displacement of Ca2+ by Na+ from the plasmalemma of root cells: A primary response to salt stress? Plant Physiol. 1985, 79, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kwak, H.W.; Lee, K.H. Effect of residual lithium ions on the structure and cytotoxicity of silk fibroin film. Int. J. Ind. Entomol. 2013, 27, 165–170. [Google Scholar] [CrossRef]
- Yin, Z.; Wu, F.; Xing, T.; Yadavalli, V.K.; Kundu, S.C.; Lu, S. A silk fibroin hydrogel with reversible sol-gel transition. RSC Adv. 2017, 7, 24085–24096. [Google Scholar] [CrossRef]
- Tiktopulo, E.I.; Kajava, A.V. Denaturation of type I collagen fibrils is an endothermic process accompanied by a noticeable change in the partial heat capacity. Biochemistry 1998, 37, 8147–8152. [Google Scholar] [CrossRef] [PubMed]
- Bozec, L.; Odlyha, M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kaplan, D.; Cebe, P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Cebe, P. Thermal properties and phase transitions in blends of Nylon-6 with silk fibroin. J. Therm. Anal. Calorim. 2008, 93, 201–206. [Google Scholar] [CrossRef]
- Lazarev, Y.A.; Grishkovsky, B.A.; Khromova, T.B. Amide I band of IR spectrum and structure of collagen and related polypeptides. Biopolymers 1985, 24, 1449–1478. [Google Scholar] [CrossRef] [PubMed]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Collagen structural hierarchy and susceptibility to degradation by ultraviolet radiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2008, 28, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Susi, H.; Ard, J.S.; Carroll, R.J. The infrared spectrum and water binding of collagen as a function of relative humidity. Biopolymers 1971, 10, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, E.; Yang, J.T. Optical rotatory dispersion and circular dichroism of the beta-form of silk fibroin in solution. Proc. Natl. Acad. Sci. USA 1966, 55, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Ayub, Z.; Arai, M.; Hirabayashi, K. Mechanism of the gelation of fibroin solution. Biosci. Biotechnol. Biochem. 1993, 57, 1910–1912. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, X.; Knight, D.P.; Zong, X.H.; Deng, F.; Yao, W.H. Effects of pH and calcium ions on the conformational transitions in silk fibroin using 2D raman correlation spectroscopy and 13C solid-state NMR. Biochemistry 2004, 43, 11302–11311. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Chen, J.; Collette, A.L.; Kim, U.J.; Altman, G.H.; Cebe, P.; Kaplan, D.L. Mechanisms of silk fibroin sol-gel transitions. J. Phys. Chem. B 2006, 110, 21630–21638. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.A.; Prausnitz, J.M.; Blanch, H.W. Protein-protein and protein-salt interactions in aqueous protein solutions containing concentrated electrolytes. Biotechnol. Bioeng. 1998, 57, 11–21. [Google Scholar] [CrossRef]
- Laos, K.; Brownsey, G.J.; Ring, S.G. Interactions between furcellaran and the globular proteins bovine serum albumin and β-lactoglobulin. Carbohydr. Polym. 2007, 67, 116–123. [Google Scholar] [CrossRef]
- Seyrek, E.; Dubin, P.L.; Tribet, C.; Gamble, E.A. Ionic strength dependence of protein-polyelectrolyte interactions. Biomacromolecules 2003, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Conio, G.; Ciferri, A.; Puett, D.; Rajagh, L. The role of pH, temperature, salt type, and salt concentration on the stability of the crystalline, helical, and randomly coiled forms of collagen. J. Biol. Chem. 1967, 242, 1361–1369. [Google Scholar] [PubMed]
- Freudenberg, U.; Behrens, S.H.; Welzel, P.B.; Müller, M.; Grimmer, M.; Salchert, K.; Taeger, T.; Schmidt, K.; Pompe, W.; Werner, C. Electrostatic interactions modulate the conformation of collagen I. Biophys. J. 2007, 92, 2108–2119. [Google Scholar] [CrossRef] [PubMed]
- Li, S.T.; Katz, E.P. An electrostatic model for collagen fibrils. The interaction of reconstituted collagen with Ca++, Na+, and Cl. Biopolymers 1976, 15, 1439–1460. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Jakus, A.E.; Shah, R.N. In situ forming collagen-hyaluronic acid membrane structures: mechanism of self-assembly and applications in regenerative medicine. Acta Biomater. 2013, 9, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Yu, T. Conformation transition of silk fibroin induced by blending chitosan. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 2293–2296. [Google Scholar] [CrossRef]
- Li, G.; Zhou, P.; Shao, Z.; Xie, X.; Chen, X.; Wang, H.; Chunyu, L.; Yu, T. The natural silk spinning process. A nucleation-dependent aggregation mechanism? Eur. J. Biochem. 2001, 268, 6600–6606. [Google Scholar] [CrossRef] [PubMed]
- Bella, J.; Brodsky, B.; Berman, H.M. Hydration structure of a collagen peptide. Structure 1995, 3, 893–906. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








| Sample | Zeta Potential (mV) |
|---|---|
| Collagen solution in acetic acid (0.5%) | 39.06 |
| Col/RSF: 75/25 | 1.41 |
| Col/RSF: 50/50 | 3.12 |
| Col/RSF: 25/75 | −0.497 |
| WCollagen (0.5%) | (dL/g) | (dL/g) | ∆[η]m | (dL/g)2 | (dL/g)2 | ∆bm* | (dL/g)2 | ∆bm** |
|---|---|---|---|---|---|---|---|---|
| 1 (Wsilk fibroin:0) | 2.48 | 38.44 | ||||||
| 0.75 | 6.47 | 2.06 | 4.41 | 72.41 | 30.04 | 42.37 | 21.64 | 50.77 |
| 0.5 | 3.54 | 1.94 | 1.6 | 75.91 | 20.87 | 55.04 | 9.67 | 66.24 |
| 0.25 | 1.78 | 1.23 | 0.55 | 39.54 | 10.94 | 28.6 | 2.54 | 37.0 |
| 0 (Wsilk fibroin:1) | 0.81 | 0.2433 |
| Sample | Zeta Potential (mV) |
|---|---|
| Silk fibroin after dialysis | −5.96 |
| Col/RSF: 75/25 (after dialysis) | 10.26 |
| Col/RSF:50/50 (after dialysis) | 9.03 |
| Col/RSF:25/75 (after dialysis) | 5.36 |
| Dialysis Days | Col/RSF:75/25 | Col/RSF:50/50 | Col/RSF: 25/75 | Water |
|---|---|---|---|---|
| Day 1 | 49.22 (s) | 50.94 (s) | 51.38 (s) | 46.58 (s) |
| Day 2 | 48.59 (s) | 49.32 (s) | 49.95 (s) | 46.58 (s) |
| Day 3 | 47.21 (s) | 48.26 (s) | 49.65 (s) | 46.58 (s) |
| Wavenumber (cm−1) | |||
|---|---|---|---|
| Amide I | Amide II | Amide III | |
| Collagen | 1634 | 1551 | 1238 |
| Col/RSF: 75/25 | 1626 | 1531 | 1236 |
| Col/RSF: 50/50 | 1644 | 1531 | 1237 |
| Col/RSF: 25/75 | 1623 | 1525 | 1234 |
| RSF | 1644 | 1531 | 1237 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaeli, I.; De Moraes, M.A.; Beppu, M.M.; Lewandowska, K.; Sionkowska, A.; Ferreira-da-Silva, F.; Ferraz, M.P.; Monteiro, F.J. Phase Behaviour and Miscibility Studies of Collagen/Silk Fibroin Macromolecular System in Dilute Solutions and Solid State. Molecules 2017, 22, 1368. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22081368
Ghaeli I, De Moraes MA, Beppu MM, Lewandowska K, Sionkowska A, Ferreira-da-Silva F, Ferraz MP, Monteiro FJ. Phase Behaviour and Miscibility Studies of Collagen/Silk Fibroin Macromolecular System in Dilute Solutions and Solid State. Molecules. 2017; 22(8):1368. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22081368
Chicago/Turabian StyleGhaeli, Ima, Mariana A. De Moraes, Marisa M. Beppu, Katarzyna Lewandowska, Alina Sionkowska, Frederico Ferreira-da-Silva, Maria P. Ferraz, and Fernando J. Monteiro. 2017. "Phase Behaviour and Miscibility Studies of Collagen/Silk Fibroin Macromolecular System in Dilute Solutions and Solid State" Molecules 22, no. 8: 1368. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22081368