A New Erythrinan Alkaloid Glycoside from the Seeds of Erythrina crista-galli
Abstract
:1. Introduction
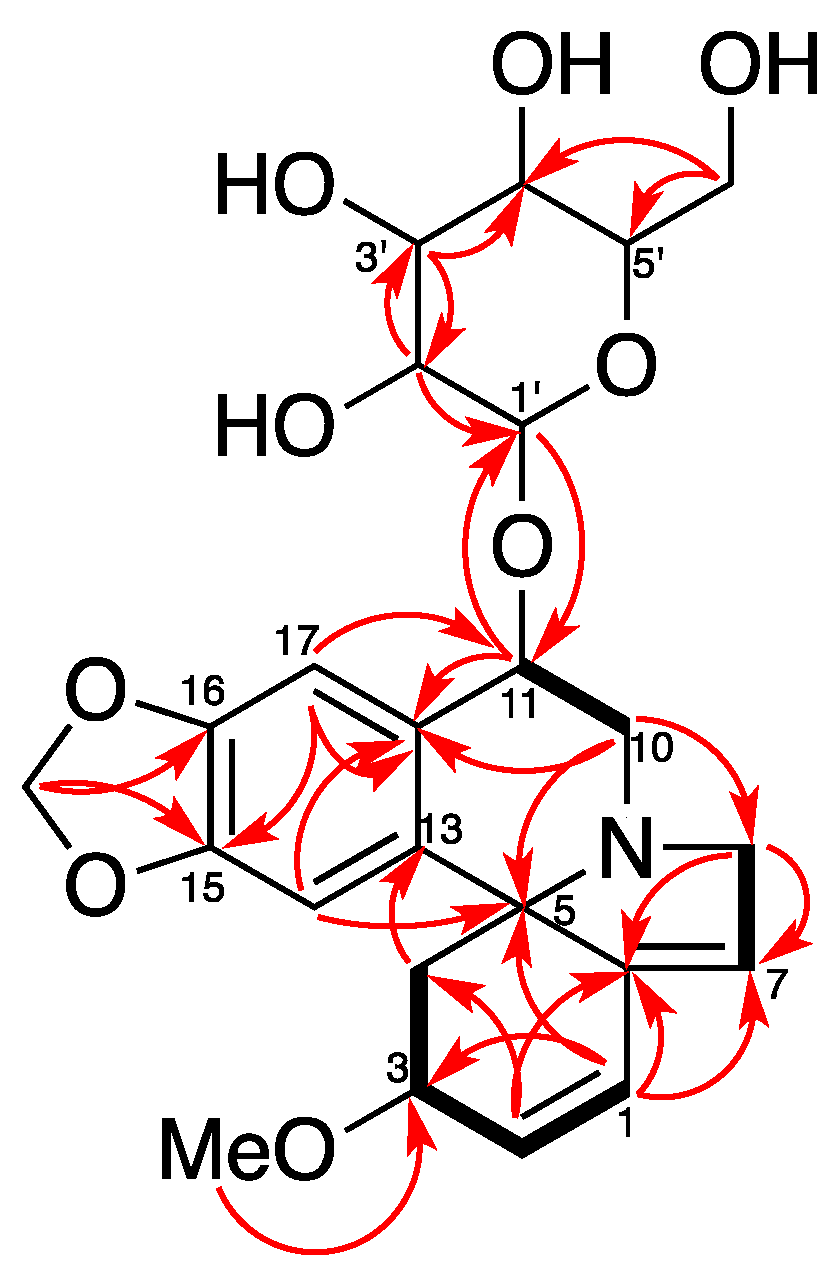
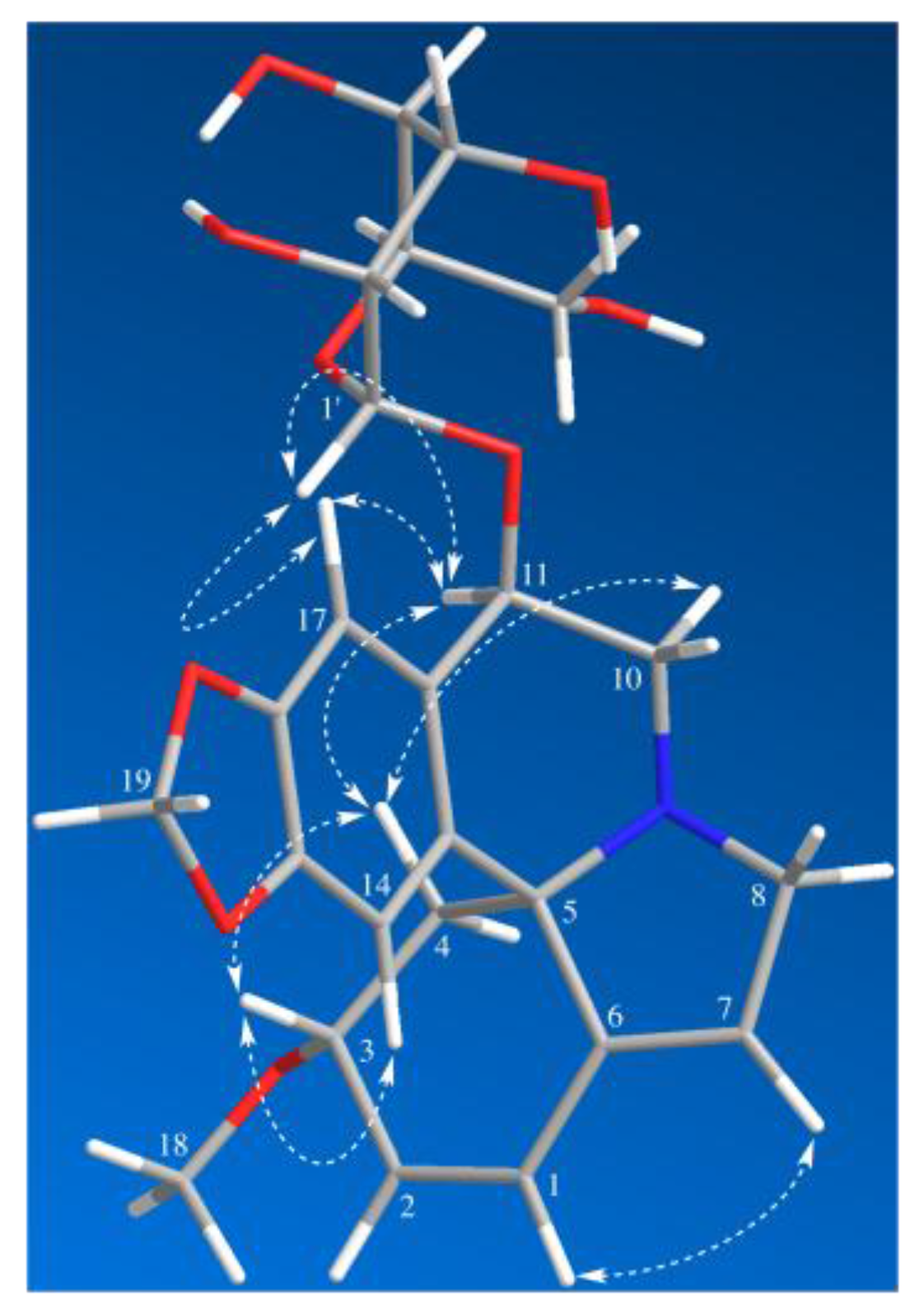
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Anti-TMV Assay
3.4.1. Virus and Host Plant
3.4.2. Leaf-Disc Method
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, B.J.; Wu, B.; Bao, M.F.; Ni, L.; Cai, X.H. New dimeric and trimeric Erythrina alkaloids from Erythrina variegata. RSC Adv. 2016, 6, 87863–87868. [Google Scholar] [CrossRef]
- Maier, U.H.; Rödl, W.; Deus-Neumann, B.; Zenk, M.H. Biosynthesis of Erythrina alkaloids in Erythrina crista-galli. Phytochemistry 1999, 52, 372–382. [Google Scholar] [CrossRef]
- Ito, K.; Haruna, M.; Jinno, Y.; Furukawa, H. Studies on the Erythrina alkaloids. XI. Alkaloids of Erythrina crysta-galli. Linn. Structure of new alkaloids, Crystamidine. Chem. Pharm. Bull. 2008, 24, 52–55. [Google Scholar] [CrossRef]
- Chawla, A.S.; Gupta, M.P.; Jackson, A.H. Alkaloidal constituents of Erythrina crista-galli flowers. J. Nat. Prod. 1987, 50, 1146–1148. [Google Scholar] [CrossRef]
- Ju-Ichi, M.; Fujitani, Y.; Furukawa, H. Structure of cristadine—A new benzylisoqunoline alkaloid. Heterocycles 1982, 19, 849–850. [Google Scholar] [CrossRef]
- Ozawa, M.; Kawamata, S.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kishida, A.; Kuroda, C.; Ohsaki, A. Structures of new Erythrinan alkaloids and nitric oxide production inhibitors from Erythrina crista-galli. Chem. Pharm. Bull. 2010, 58, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Tanaka, T.; Etoh, H. Three pterocarpans from Erythrina crista-galli. Phytochemistry 1997, 45, 835–838. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Ward, J.A.; Drake, S.; Rao, G.S. Antimicrobial agents from higher plants. Erycristagallin, a new pterocarpene from the roots of the bolivian coral tree, Erythrina crista-galli. Heterocycles 1984, 22, 1673–1675. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Gollapudi, S.R.; Gerlach, D.C.; Drake, S.D.; Véliz, E.A.; Ward, J.A. Erycristin, a new antimicrobial petrocarpan from Erythrina crista-galli. Phytochemistry 1988, 27, 381–385. [Google Scholar] [CrossRef]
- Iinuma, M.; Okawa, Y.; Tanaka, T. Three new cinnamylphenols in heartwood of Erythrina crista-galli. Phytochemistry 1994, 37, 1153–1155. [Google Scholar] [CrossRef]
- Ingham, J.L.; Markham, K.R. Identification of the Erythrina phytoalexin cristacarpin and a note on the chirality of other 6α-hydroxypterocarpans. Phytochemistry 1980, 19, 1203–1207. [Google Scholar] [CrossRef]
- Chawla, A.S.; Chunchatprasert, S.; Jackson, A.H. Studies of Erythrina alkaloids: VII–13C-NMR spectral studies of some Erythina alkaloids. Org. Magn. Reson. 1983, 21, 39–41. [Google Scholar] [CrossRef]
- Barton, D.H.; James, R.; Kirby, G.W.; Turner, D.W.; Widdowson, D.A. Phenol oxidation and biosynthesis. Part XVIII. The structure and biosynthesis of Erythrina alkaloids. J. Chem. Soc. C Org. 1968, 12, 1529–1537. [Google Scholar] [CrossRef]
- Amer, M.E.; El-Masry, S. Three novel glycodienoid alkaloids from Erythrina lysistemon. J. Nat. Prod. 1991, 54, 161–166. [Google Scholar] [CrossRef]
- Wanjala, C.C.W.; Majinda, R.T. Two novel glucodienoid alkaloids from Erythrina latissima seeds. J. Nat. Prod. 2000, 63, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Honda, K.; Nakai, I.; Kishida, A.; Ohsaki, A. Hypaphorine, an indole alkaloid from Erythrina velutina, induced sleep on normal mice. Bioorg. Med. Chem. Lett. 2008, 18, 3992–3994. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, T.; Innok, P.; Suksamrarn, A. Erythrina alkaloids and a pterocarpan from the bark of Erythrina subumbrans. J. Nat. Prod. 2008, 71, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, X.H.; Dong, J.H.; Sang, P.; Fang, X.; Di, Y.T.; Zhang, Z.K.; Hao, X.J. Tobacco mosaic virus (TMV) inhibitors from Picrasma quassioides Benn. J. Agric. Food Chem. 2009, 57, 6590–6595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Fan, H.J.; Li, Y.; Shi, Z.L.; Pan, Y.; Lu, C.P. Development of a multi-mimotope peptide as a vaccine immunogen for infectious bursal disease virus. Vaccine 2007, 25, 4447–4455. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| C-1 | 126.2 | 6.59 (1H, dd, J = 10.2, 2.1 Hz) | 125.4 | 6.53 (1H, dd, J = 10.1, 2.3 Hz) |
| C-2 | 132.7 | 6.04 (1H, d, J = 10.2 Hz) | 131.6 | 5.97 (1H, d, J = 10.1 Hz) |
| C-3 | 77.4 | 4.01 (1H, m) | 76.2 | 3.96 (1H, m) |
| C-4 | 41.2 | 2.46 (1H, dd, J = 11.5, 5.5 Hz), 1.73 (1H, dd, J = 11.5, 10.4 Hz) | 42.0 | 2.49 (1H, dd, J = 11.5, 5.5 Hz) 1.83 (1H, dd, J = 11.5, 10.4 Hz) |
| C-5 | 67.8 | □ | 67.5 | □ |
| C-6 | 143.7 | □ | 142.4 | □ |
| C-7 | 124.5 | 5.78 (1H, br s) | 123.1 | 5.72 (1H, br s, 1H) |
| C-8 | 59.3 | 3.89 (1H, br d, J = 15.5 Hz) 3.70 (1H, dd, J = 15.5, 3.0 Hz) | 57.7 | 3.72 (1H, dd, J = 14.5, 3.0 Hz) 3.53–3.45 (1H, overlap) |
| C-10 | 50.4 | 3.57 (1H, dd, J = 14.1, 4.8 Hz) 3.37–3.30 (1H, overlap) | 44.6 | 3.53–3.45 (1H, overlap) 2.92–2.83 (1H, overlap) |
| C-11 | 74.5 | 4.70 (1H, t, J = 4.3 Hz) | 25.3 | 2.92–2.83 (1H, overlap) 2.71–2.64 (1H, m) |
| C-12 | 129.1 | □ | 128.1 | □ |
| C-13 | 132.0 | □ | 132.7 | □ |
| C-14 | 106.3 | 6.73 (1H, s) | 106.3 | 6.62 (1H, s) |
| C-15 | 148.9 | □ | 146.2 | □ |
| C-16 | 148.1 | □ | 145.9 | □ |
| C-17 | 109.7 | 7.15 (1H, s) | 108.8 | 6.76 (1H, s) |
| C-18 | 56.6 | 3.34 (3H, s) | 56.1 | 3.32 (3H, s) |
| C-19 | 102.3 | 5.91 (1H, d, J = 1.2 Hz) 5.90 (1H, d, J = 1.2 Hz) | 100.8 | 5.90 (1H, d, J = 1.5 Hz) 5.87 (1H, d, J = 1.5 Hz) |
| C-1′ | 105.9 | 4.61 (1H, d, J = 7.8 Hz) | □ | □ |
| C-2′ | 75.4 | 3.23 (1H, dd, J = 9.2, 7.8 Hz) | □ | □ |
| C-3′ | 78.2 | 3.42 (1H, t, J = 8.8 Hz) | □ | □ |
| C-4′ | 71.6 | 3.39–3.28 (1H, overlap) | □ | □ |
| C-5′ | 78.0 | 3.39–3.28 (1H, overlap) | □ | □ |
| C-6′ | 62.8 | 3.92 (1H, dd, J = 11.8, 2.0 Hz) 3.73 (1H, dd, J = 11.8, 5.4 Hz) | □ | □ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Q.-W.; Ni, J.-C.; Fang, P.-H.; Chen, Q.-J. A New Erythrinan Alkaloid Glycoside from the Seeds of Erythrina crista-galli. Molecules 2017, 22, 1558. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22091558
Tan Q-W, Ni J-C, Fang P-H, Chen Q-J. A New Erythrinan Alkaloid Glycoside from the Seeds of Erythrina crista-galli. Molecules. 2017; 22(9):1558. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22091558
Chicago/Turabian StyleTan, Qing-Wei, Jian-Cheng Ni, Pei-Hua Fang, and Qi-Jian Chen. 2017. "A New Erythrinan Alkaloid Glycoside from the Seeds of Erythrina crista-galli" Molecules 22, no. 9: 1558. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22091558





