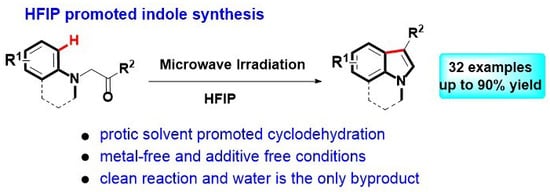
HFIP-Promoted Bischler Indole Synthesis under Microwave Irradiation
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Indoles and Pyrrolo[3,2,1-ij]quinolones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; Aiello, A.; D’Aniello, F.; Senese, M.; Menna, M. Alkaloids from marine invertebrates as important leads for anticancer drugs discovery and development. Molecules 2014, 19, 20391–20423. [Google Scholar] [CrossRef] [PubMed]
- Bariwal, J.; Voskressensky, L.G.; van der Eycken, E.V. Recent advances in spirocyclization of indole derivatives. Chem. Soc. Rev. 2018, 47, 3831–3848. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef] [PubMed]
- Dreinert, A.; Wolf, A.; Mentzel, T.; Meunier, B.; Fehr, M. The cytochrome bc1 complex inhibitor ametoctradin has an unusual binding mode. BBA-Bioenerg. 2018, 1859, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Fuenfschilling, P.C.; Hoehn, P.; Mutz, J.-P. An improved manufacturing process for fluvastatin. Org. Process Res. Dev. 2007, 11, 13–18. [Google Scholar] [CrossRef]
- Inman, M.; Moody, C.J. Indole synthesis-something old, something new. Chem. Sci. 2013, 4, 29–41. [Google Scholar] [CrossRef]
- Vicente, R. Recent advances in indole syntheses: New routes for a classic target. Org. Biomol. Chem. 2011, 9, 6469–6480. [Google Scholar] [CrossRef]
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef]
- Buu-Hoï, N.P.; Saint-Ruf, G.; Deschamps, D.; Hieu, H.T. Carcinogenic nitrogen compounds. Part LXXII. The Möhlau-Bischler reaction as a preparative route to 2-arylindoles. J. Chem. Soc. C Org. 1971, 2606–2609. [Google Scholar] [CrossRef]
- Bigot, P.; Saint-Ruf, G.; Buu-Hoï, N.P. Carcinogenic nitrogen compounds. Part LXXXII. Polycyclic indoles by means of the Möhlau-Bischler synthesis. J. Chem. Soc. Perkin Trans. 1 1972, 2573–2576. [Google Scholar] [CrossRef]
- Robinson, B. The fischer indole synthesis. Chem. Rev. 1963, 63, 373–401. [Google Scholar] [CrossRef]
- Heravi, M.M.; Rohani, S.; Zadsirjan, V.; Zahedi, N. Fischer indole synthesis applied to the total synthesis of natural products. RSC Adv. 2017, 7, 52852–52887. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, G.; Palmieri, G.; Bosco, M.; Dalpozzo, R. The reaction of vinyl grignard reagents with 2-substituted nitroarenes: A new approach to the synthesis of 7-substituted indoles. Tetrahedron Lett. 1989, 30, 2129–2132. [Google Scholar] [CrossRef]
- Bartoli, G.; Dalpozzo, R.; Nardi, M. Applications of Bartoli indole synthesis. Chem. Soc. Rev. 2014, 43, 4728–4750. [Google Scholar] [CrossRef] [PubMed]
- Bashford, K.E.; Cooper, A.L.; Kane, P.D.; Moody, C.J.; Muthusamy, S.; Swann, E. N-H Insertion reactions of rhodium carbenoids. Part 3.1. The development of a modified Bischler indole synthesis and a new protecting-group strategy for indoles. J. Chem. Soc. Perkin Trans. 1 2002, 1672–1687. [Google Scholar] [CrossRef]
- Tsuchikama, K.; Hashimoto, Y.-K.; Endo, K.; Shibata, T. Iridium-catalyzed selective synthesis of 4-substituted benzofurans and indoles via directed cyclodehydration. Adv. Synth. Catal. 2009, 351, 2850–2854. [Google Scholar] [CrossRef]
- Tokunaga, M.; Ota, M.; Haga, M.-A.; Wakatsuki, Y. A practical one-pot synthesis of 2,3-disubstituted indoles from unactivated anilines. Tetrahedron Lett. 2001, 42, 3865–3868. [Google Scholar] [CrossRef]
- Kumar, M.P.; Liu, R.-S. Zn(OTf)2-catalyzed cyclization of proparyl alcohols with anilines, phenols, and amides for synthesis of indoles, benzofurans, and oxazoles through different annulation mechanisms. J. Org. Chem. 2006, 71, 4951–4955. [Google Scholar] [CrossRef]
- Bunescu, A.; Piemontesi, C.; Wang, Q.; Zhu, J. Heteroannulation of arynes with N-aryl-α-aminoketones for the synthesis of unsymmetrical N-aryl-2,3-disubstituted indoles: An aryne twist of Bischler-Möhlau indole synthesis. Chem. Commun. 2013, 49, 10284–10286. [Google Scholar] [CrossRef] [PubMed]
- MacDonough, M.T.; Shi, Z.; Pinney, K.G. Mechanistic considerations in the synthesis of 2-aryl-indole analogues under Bischler-Mohlau conditions. Tetrahedron Lett. 2015, 56, 3624–3629. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Perumal, S.; Avendaño, C.; Menéndez, J.C. Microwave-assisted, solvent-free Bischler indole synthesis. Synlett 2006, 91–95. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Gao, Y.-C.; Zheng, W.; Tang, R.-Y. Oxidative radical cyclization of N-methyl-N-arylpropiolamide to isatins via cleavage of the carbon-carbon triple bond. Adv. Synth. Catal. 2018, 360, 3391–3400. [Google Scholar] [CrossRef]
- Tang, R.-Y.; Guo, X.-K.; Xiang, J.-N.; Li, J.-H. Palladium-catalyzed synthesis of 3-acylated indoles involving oxidative cross-coupling of indoles with α-amino carbonyl compounds. J. Org. Chem. 2013, 78, 11163–11171. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zhang, J.-R.; Liao, Y.-Y.; Xu, L.; Hu, M.; Tang, R.-Y. CuCl/air-mediated oxidative coupling reaction of imidazoheterocycles with N-aryl glycine esters. RSC Adv. 2017, 7, 30152–30159. [Google Scholar] [CrossRef]
- Ji, X.-M.; Zhou, S.-J.; Deng, C.-L.; Chen, F.; Tang, R.-Y. NH4PF6-promoted cyclodehydration of α-amino carbonyl compounds: Efficient synthesis of pyrrolo [3,2,1-ij] quinoline and indole derivatives. RSC Adv. 2014, 4, 53837–53841. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, R.; Miller, D.D. Microwave-assisted synthesis of medicinally relevant indoles. Curr. Med. Chem. 2011, 18, 615–637. [Google Scholar] [CrossRef]
- Driowya, M.; Saber, A.; Marzag, H.; Demange, L.; Benhida, R.; Bougrin, K. Microwave-assisted synthesis of bioactive six-membered heterocycles and their fused analogues. Molecules 2016, 21, 492. [Google Scholar] [CrossRef]
- Brancale, A.; Silvestri, R. Indole, a core nucleus for potent inhibitors of tubulin polymerization. Med. Res. Rev. 2007, 27, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.L.; Zhang, W.M.; Lv, P.C.; Zhu, H.L. Indole-based, Antiproliferative Agents Targeting Tublin Polymerizaton. Curr. Top. Med. Chem. 2017, 17, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Sakami, S.; Kawai, K.; Maeda, M.; Aoki, T.; Fujii, H.; Ohno, H.; Ito, T.; Saitoh, A.; Nakao, K.; Izumimoto, N.; et al. Design and synthesis of a metabolically stable and potent antitussive agent, a novel δ opioid receptor antagonist, TRK-851. Bioorg. Med. Chem. 2008, 16, 7956–7967. [Google Scholar] [CrossRef] [PubMed]
- Matesic, L.; Locke, J.M.; Vine, K.L.; Ranson, M.; Bremner, J.B.; Skropeta, D. Synthesis and anti-leukaemic activity of pyrrolo[3,2,1-ij]indole-1,2-diones, pyrrolo[3,2,1-ij]quinoline-1,2-diones and other polycyclic isatin derivatives. Tetrahedron 2012, 68, 6810–6819. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; Feng, C.; Liu, Z.; Bai, E.; Wang, X.; Lei, M.; Cheng, H.; Feng, H.; Shi, J.; et al. Design, synthesis, SAR discussion, in vitro and in vivo evaluation of novel selective EGFR modulator to inhibit L858R/T790M double mutants. Eur. J. Med. Chem. 2017, 135, 12–23. [Google Scholar] [CrossRef]
- Phipps, R.J.; Grimster, N.P.; Gaunt, M.J. Cu(II)-catalyzed direct and site-selective arylation of indoles under mild conditions. J. Am. Chem. Soc. 2008, 130, 8172–8174. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, K.; Tanaka, S.; Konno, M.; Onozawa, S.; Chiba, M.; Tanaka, Y.; Sasaki, Y.; Okubo, R.; Hattori, T. Me2AlCl-mediated carboxylation, ethoxycarbonylation, and carbamoylation of indoles. Tetrahedron 2016, 72, 734–745. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2–22, 24–34 are available from the authors. |



| Entry | Solvent | Time/(min) | T/°C | Yield (%) b |
|---|---|---|---|---|
| 1 | HFIP | 30 | 120 | 76 |
| 2 | CF3CH2OH | 30 | 120 | 45 |
| 3 | i-PrOH | 30 | 120 | 21 |
| 4 | EtOH | 30 | 120 | 23 |
| 5 | HFIP | 30 | 80 | 51 |
| 6 | HFIP | 30 | 100 | 72 |
| 7 | HFIP | 20 | 120 | 58 |
| 8 | HFIP | 40 | 120 | 88 |
| 9 c | HFIP | 960 | 120 | 86 |

 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, G.; Zhang, Z.-X.; Zhang, C.-B.; Xu, H.-H.; Tang, R.-Y. HFIP-Promoted Bischler Indole Synthesis under Microwave Irradiation. Molecules 2018, 23, 3317. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123317
Yao G, Zhang Z-X, Zhang C-B, Xu H-H, Tang R-Y. HFIP-Promoted Bischler Indole Synthesis under Microwave Irradiation. Molecules. 2018; 23(12):3317. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123317
Chicago/Turabian StyleYao, Guangkai, Zhi-Xiang Zhang, Cheng-Bei Zhang, Han-Hong Xu, and Ri-Yuan Tang. 2018. "HFIP-Promoted Bischler Indole Synthesis under Microwave Irradiation" Molecules 23, no. 12: 3317. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123317