Comparative Evaluation of Soluble and Insoluble-Bound Phenolics and Antioxidant Activity of Two Chinese Mistletoes
Abstract
:1. Introduction
2. Results and Discussion
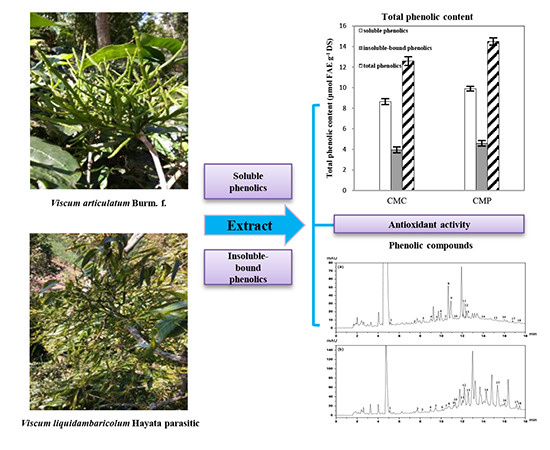
2.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.2. Antioxidant Activities In Vitro
2.3. Identification and Quantification of the Two Chinese Mistletoe (CM) Extracts by High-Performance Liquid Chromatography (HPLC)
3. Materials and Methods
3.1. Materials and Chemical Reagent
3.2. Separation of Phenolic Compounds
3.3. Determination of TPC
3.4. Determination of TFC
3.5. Determination of Ferric Reducing Antioxidant Power (FRAP)
3.6. Determination of H2O2 Scavenging Activity (HPSA)
3.7. Determination of DPPH Radical Scavenging Activity (DRSA)
3.8. Determination of Trolox Equivalent Antioxidant Capacity (TEAC)
3.9. HPLC Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nickrent, D.L. Santalales (Including Mistletoes). In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar] [CrossRef]
- Nazaruk, J.; Orlikowski, P. Phytochemical profile and therapeutic potential of Viscum album L. Nat. Prod. Res. 2015, 30, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Quispe, C.; Areche, C.; Sepúlveda, B. Phenolic Compounds in Chilean Mistletoe (Quintral, Tristerix tetrandus) Analyzed by UHPLC-Q/Orbitrap/MS/MS and Its Antioxidant Properties. Molecules 2016, 21, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.; Végvári, Z. Bioclimatic constrains of European Mistletoe Viscum album at its southern distribution limit on past and present temporal scales, Pannon region, Hungary. Clim. Res. 2017, 71, 237–248. [Google Scholar] [CrossRef]
- Yoo, J.M.; Yang, J.H.; Kim, Y.S.; Yang, H.J.; Cho, W.K.; Ma, J.Y. Inhibitory Effects of Viscum coloratum Extract on IgE/Antigen-Activated Mast Cells and Mast Cell-Derived Inflammatory Mediator-Activated Chondrocytes. Molecules 2017, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Rajabalaya, R.; Lee, S.H.F.; Tennakoon, K.U.; Le, Q.V.; Idris, A.; Zulkipli, I.N.; Keasberry, N.; David, S.R. Parasitic Mistletoes of the Genera Scurrula and Viscum: From Bench to Bedside. Molecules 2016, 21, 1048–1081. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Cheng, W.Z.; Gong, F.; Ma, A.L.; Yu, Q.W.; Zhang, J.Y.; Hu, C.Y.; Chen, X.H.; Zhang, D.Q. Active Chinese mistletoe lectin-55 enhances colon cancer surveillance through regulating innate and adaptive immune responses. World J. Gastroenterol. 2008, 14, 5274–5281. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.P.; Singh, P.K. Viscum articulatum Burm. f.: A review on its phytochemistry, pharmacology and traditional uses. J. Pharm. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.; Nowak, R.; Gawlik-Dziki, U.; Lemieszek, M.K.; Rzeski, W. LC-ESI-MS/MS Identification of Biologically Active Phenolic Compounds in Mistletoe Berry Extracts from Different Host Trees. Molecules 2017, 22, 624. [Google Scholar] [CrossRef] [PubMed]
- Luczkiewicz, M.; Cisowski, W.; Kaiser, P.; Ochocka, R.; Piotrowski, A. Comparative analysis of phenolic acids in mistletoe plants from various hosts. Acta Pol. Pharm. 2001, 58, 373–379. [Google Scholar] [PubMed]
- Geetha, K.M.; Bindu, V.; Murugan, V. Antioxidant potential of Viscum articulatum burm. Chron. Young Sci. 2013, 4, 32–35. [Google Scholar] [CrossRef]
- Li, H.Z.; Hou, Z.; Li, C.; Zhang, Y.; Shen, T.; Hu, Q.W.; Ren, D.M. Three pairs of diastereoisomeric flavanone glycosides from Viscum articulatum. Fitoterapia 2015, 102, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.L.; Huang, N.; Zheng, Y.T.; Yang, Y.P.; Li, X.L. Two New Phenolic Glycosides from Viscum articulatum. Molecules 2008, 13, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- Leu, Y.L.; Kuo, S.M.; Hwang, T.L.; Chiu, S.T. The inhibition of superoxide anion generation by neutrophils from Viscum articulactum. Chem. Pharm. Bull. 2004, 52, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Zhang, X.; Chen, G.L.; Yu, J.; Yang, L.Q.; Gao, Y.Q. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. J. Funct. Foods 2016, 24, 359–372. [Google Scholar] [CrossRef]
- Lewandowska, H.; Kalinowska, M.; Lewandowski, W.; Stępkowski, T.M.; Brzóska, K. The role of natural polyphenols in cell signaling and cytoprotection against cancer development. J. Nutr. Biochem. 2016, 32, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Laus, M.N.; Benedetto, N.A.D.; Caporizzi, R.; Tozzi, D.; Soccio, M.; Giuzio, L.; Vita, P.D.; Flagella, Z.; Pastore, D. Evaluation of Phenolic Antioxidant Capacity in Grains of Modern and Old Durum Wheat Genotypes by the Novel QUENCHERABTS Approach. Plant Foods Hum. Nutr. 2015, 70, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J.D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216–1237. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vasanthan, T.; Temelli, F. Analysis of Phenolic Acids in Barley by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2001, 49, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Content of Insoluble Bound Phenolics in Millets and Their Contribution to Antioxidant Capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Inhibitory Activities of Soluble and Bound Millet Seed Phenolics on Free Radicals and Reactive Oxygen Species. J. Agric. Food Chem. 2011, 59, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Köksal, E.; Bursal, E.; Gülçin, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.C.; Alwasel, S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Rishmawi, S.; Ariqat, S.H.; Khalid, M.F.; Warad, I.; Salah, Z. Anticancer Activity, Antioxidant Activity, and Phenolic and Flavonoids Content of Wild Tragopogon porrifolius Plant Extracts. Evid.-Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Yuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Quan, N.V.; Anh, T.T.A.; Anh, L.H.; Minh, T.N. Phenolic Compositions and Antioxidant Properties in Bark, Flower, Inner Skin, Kernel and Leaf Extracts of Castanea crenata Sieb. et Zucc. Antioxidants 2017, 6, 31. [Google Scholar] [CrossRef]
- Song, F.L.; Gan, R.Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.B. Total Phenolic Contents and Antioxidant Capacities of Selected Chinese Medicinal Plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods 2013, 5, 1191–1203. [Google Scholar] [CrossRef]
- Minh, T.N.; Khang, D.T.; Tuyen, P.H.; Minh, L.T.; Anh, L.H.; Quan, N.V.; Ha, P.T.T.; Quan, N.T.; Toan, N.P.; Elzaawely, A.A.; et al. Phenolic Compounds and Antioxidant Activity of Phalaenopsis Orchid Hybrids. Antioxidants 2016, 5, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.N.; Tuyen, P.T.; Khang, D.T.; Quan, N.V.; Ha, P.T.T.; Quan, N.T.; Andriana, Y.; Fan, X.; Van, T.M.; Khanh, T.D.; et al. Potential Use of Plant Waste from the Moth Orchid (Phalaenopsis Sogo Yukidian “V3”) as an Antioxidant Source. Foods 2017, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Issaad, F.Z.; Fernandes, I.P.G.; Enache, T.A.; Mouats, C.; Rodrigues, I.A.; Oliveira-Brett, A.M. Flavonoids in Selected Mediterranean Fruits: Extraction, Electrochemical Detection and Total Antioxidant Capacity Evaluation. Electroanalysis 2016, 29, 358–366. [Google Scholar] [CrossRef]
- Hermans, N.; Cos, P.; Maes, L.; De, B.T.; Vanden, B.D.; Vlietinck, A.J.; Pieters, L. Challenges and Pitfalls in Antioxidant Research. Curr. Med. Chem. 2007, 14, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Jiménez, R.; Aruoma, O.I.; Hercberg, S.; Sánchez-garcía, I.; Fraga, C.; Serramajem, L.; Ngo, J.; Aranceta, J.; Solomons, N.W. Aspects of antioxidant foods and supplements in health and disease. Nutr. Rev. 2009, 67, S140–S144. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.N.; Lin, Y.S.; Hsu, Y.S.; Chiu, E.M.; Ho, C.T. Soluble and insoluble phenolic compounds and antioxidant activity of immature calamondin affected by solvents and heat treatment. Food Chem. 2014, 161, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.N.S.; Saraswathy, A.; Amerjothy, S.; Susan, T.; Ravishankar, B. Total Phenol Content and In Vitro Antioxidant Potential of Helicanthus elastica (Desr.) Danser-A Less-explored Indian Mango Mistletoe. J. Tradit. Complement. Med. 2014, 4, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, C.T.; Balachandran, I. Total Phenolics and Total Flavonoids in Selected Indian Medicinal Plants. Indian J. Pharm. Sci. 2012, 74, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, S.I.; Ishimaru, K.; Hou, D.X.; Hayashi, N. Antioxidant Activity and Phenolic Content of Mistletoe Extracts Following High-Temperature Batch Extraction. Food Sci. Technol. Res. 2014, 20, 201–206. [Google Scholar] [CrossRef]
- Meng, Y.; Su, A.; Yuan, S.; Zhao, H.; Tan, S.; Hu, C.; Deng, H.; Guo, Y. Evaluation of Total Flavonoids, Myricetin, and Quercetin from Hovenia dulcis Thunb. As Inhibitors of α-Amylase and α-Glucosidase. Plant Foods Hum. Nutr. 2016, 71, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J. Funct. Foods 2011, 3, 144–158. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Kern, S.M.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M. Absorption of hydroxycinnamates in humans after high-bran cereal consumption. J. Agric. Food Chem. 2003, 51, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Naczk, M.; Shahidia, F. Effect of processing on the antioxidant activity of millet grains. Food Chem. 2012, 133, 1–9. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: the FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Carvajal, A.; Bernal-Martínez, L.R.; García-Gasca, M.T.; Dominguez-Lopez, A. In vitro gastrointestinal digestion of Hibiscus sabdariffa L.: The use of its natural matrix to improve the concentration of phenolic compounds in gut. Food Sci. Technol. 2013, 51, 260–265. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Shahidi, F. Scavenging of reactive-oxygen species and DPPH free radicals by extracts of borage and evening primrose meals. Food Chem. 2000, 70, 17–26. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Riceevans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
Sample Availability: Samples of the plant materials and extracts are available from the authors. |



| Plant Material | CMC | CMP |
|---|---|---|
| Ferric Reducing Antioxidant Power (μmol FE/g DS) | ||
| Soluble | 42.25 ± 1.49a 2 | 44.76 ± 0.32a 1 |
| Insoluble-bound | 8.07 ± 0.75b 2 | 10.31 ± 0.46b 1 |
| Hydrogen Peroxide Scavenging Activity (μmol FAE/g DS) | ||
| Soluble | 1429.34 ± 7.69a 1 | 1431.87 ± 4.16a 1 |
| Insoluble-bound | 1383.79 ± 3.33b 1 | 1231.67 ± 12.23b 2 |
| DPPH Radical Scavenging Activity (μmol FAE/g DS) | ||
| Soluble | 2.19 ± 0.11a 2 | 2.51 ± 0.04a 1 |
| Insoluble-bound | 1.51 ± 0.07b 2 | 1.83 ± 0.09b 1 |
| Trolox Equivalent Antioxidant Capacity (μmol TE/g DS) | ||
| Soluble | 81.03 ± 0.90a 2 | 84.92 ± 1.50a 1 |
| Insoluble-bound | 5.78 ± 1.24b 1 | 1.40 ± 0.24b 2,* |
| Phenolic Compounds | CMC | CMP |
|---|---|---|
| Hydroxybenzoic Acids | ||
| Gallic acid | 67.51 ± 12.21 | 172.65 ± 4.34 |
| Protocatechuic acid | 39.65 ± 9.27 | 203.23 ± 12.89 |
| p-Hydroxybenzoic acid | 133.88 ± 116.31 | 787.95 ± 138.89 |
| Vanillic acid | 1325.77 ± 23.34 | 408.82 ± 29.21 |
| Syringic acid | 432.33 ± 370.24 | 66.68 ± 27.90 |
| Vanillin | 70.08 ± 36.40 | 136.92 ± 130.03 |
| Total | 2069.23 | 1776.25 |
| Hydroxycinnamic Acids | ||
| Chlorogenic acid | 93.36 ± 50.71 | 68.85 ± 27.70 |
| Caffeic acid | 105.59 ± 12.03 | 119.18 ± 16.77 |
| p-Coumaric acid | 85.05 ± 70.36 | 820.10 ± 355.68 |
| Ferulic acid | 560.48 ± 25.71 | 248.82 ± 101.63 |
| trans-Cinnamic acid | 67.80 ± 9.00 | 172.60 ± 1.93 |
| Total | 912.28 | 1429.55 |
| Flavonoids | ||
| Catechin hydrate | 188.60 ± 138.25 | 200.51 ± 150.87 |
| Epicatechin | 238.18 ± 79.30 | 569.53 ± 67.92 |
| (−)-Epigallocatechin | 237.03 ± 8.36 | 542.10 ± 23.71 |
| Myricetin | 154.88 ± 62.10 | 2209.79 ± 1476.96 |
| Quercetin | 85.22 ± 28.75 | 834.71 ± 543.19 |
| Kaemferol | 40.48 ± 10.23 | 197.63 ± 157.76 |
| Apigenin | 25.50 ± 5.66 | 447.18 ± 3.32 |
| Total | 969.89 | 5001.44 * |
| Phenolic Compounds | CMC | CMP |
|---|---|---|
| Hydroxybenzoic Acids | ||
| Gallic acid | 6.34 ± 0.56 | 21.41 ± 0.92 |
| Protocatechuic acid | 27.43 ± 1.73 | 23.24 ± 4.45 |
| p-Hydroxybenzoic acid | 48.02 ± 22.44 | 55.20 ± 42.12 |
| Vanillic acid | 52.73 ± 8.40 | 37.40 ± 25.69 |
| Syringic acid | 6.39 ± 1.14 | 10.45 ± 6.34 |
| Vanillin | 13.18 ± 7.35 | 39.69 ± 2.06 |
| Total | 154.08 | 187.40 |
| Hydroxycinnamic Acids | ||
| Chlorogenic acid | 12.28 ± 2.43 | 22.21 ± 12.98 |
| Caffeic acid | 49.88 ± 2.41 | 28.20 ± 1.19 |
| p-Coumaric acid | 206.97 ± 21.39 | 14.26 ± 13.13 |
| Ferulic acid | 97.94 ± 4.63 | 171.18 ± 4.88 |
| trans-Cinnamic acid | 43.06 ± 1.02 | 124.38 ± 2.31 |
| Total | 410.12 | 360.23 |
| Flavonoids | ||
| Catechin hydrate | 129.17 ± 32.41 | 92.21 ± 2.78 |
| Epicatechin | 11.21 ± 9.76 | 26.34 ± 12.67 |
| (−)-Epigallocatechin | 14.63 ± 10.29 | 223.32 ± 24.87 |
| Myricetin | 33.14 ± 19.55 | 75.23 ± 49.31 |
| Quercetin | 41.44 ± 11.71 | 62.30 ± 31.66 |
| Kaemferol | 18.15 ± 12.05 | 99.40 ± 69.46 |
| Apigenin | 10.35 ± 9.34 | 9.33 ± 6.64 |
| Total | 258.10 | 588.13 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Yang, S.; Li, Y.; Xue, X.; Huang, Y.; Luo, H.; Zhang, Y.; Lu, Z. Comparative Evaluation of Soluble and Insoluble-Bound Phenolics and Antioxidant Activity of Two Chinese Mistletoes. Molecules 2018, 23, 359. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23020359
Li Q, Yang S, Li Y, Xue X, Huang Y, Luo H, Zhang Y, Lu Z. Comparative Evaluation of Soluble and Insoluble-Bound Phenolics and Antioxidant Activity of Two Chinese Mistletoes. Molecules. 2018; 23(2):359. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23020359
Chicago/Turabian StyleLi, Qing, Shihua Yang, Yongqiang Li, Xiaofeng Xue, Yonghua Huang, Hengguo Luo, Yiming Zhang, and Zhichao Lu. 2018. "Comparative Evaluation of Soluble and Insoluble-Bound Phenolics and Antioxidant Activity of Two Chinese Mistletoes" Molecules 23, no. 2: 359. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23020359