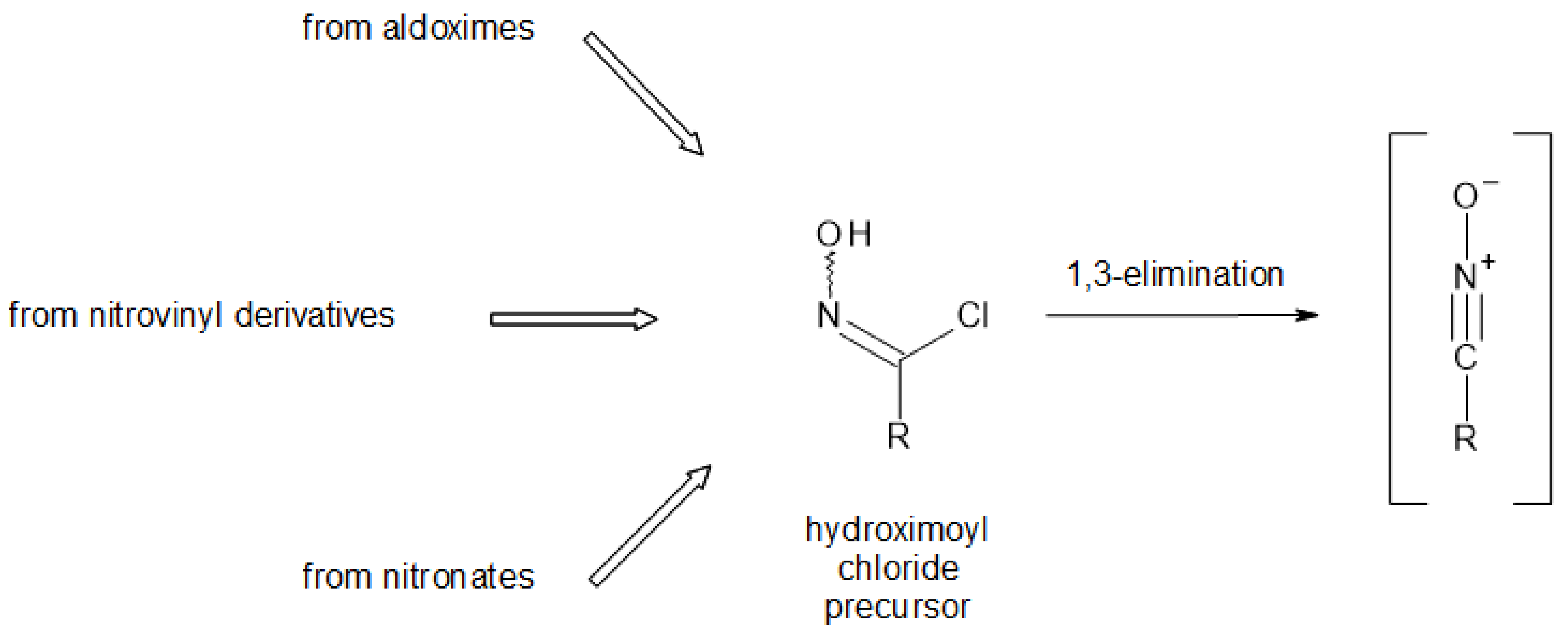
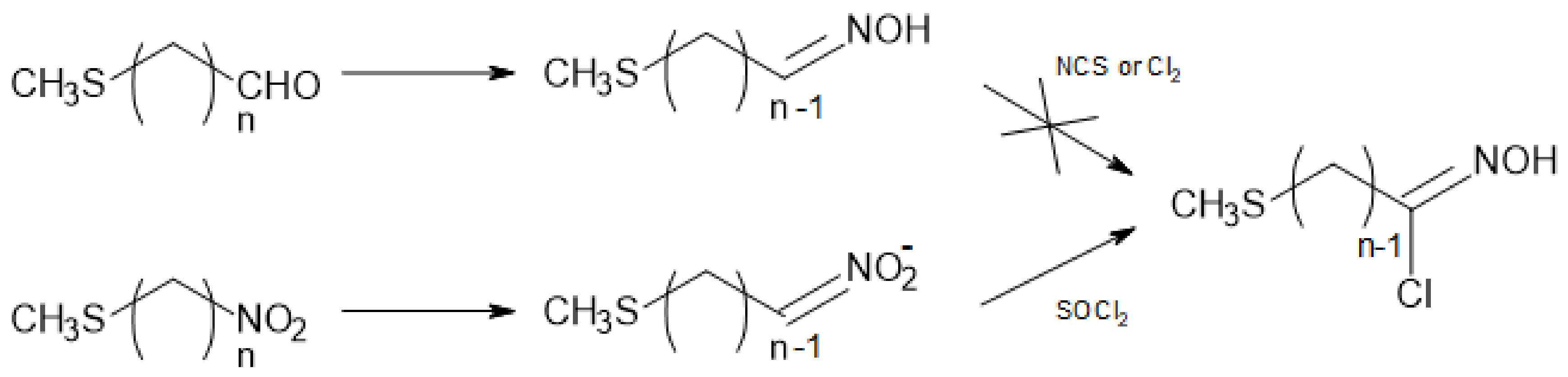
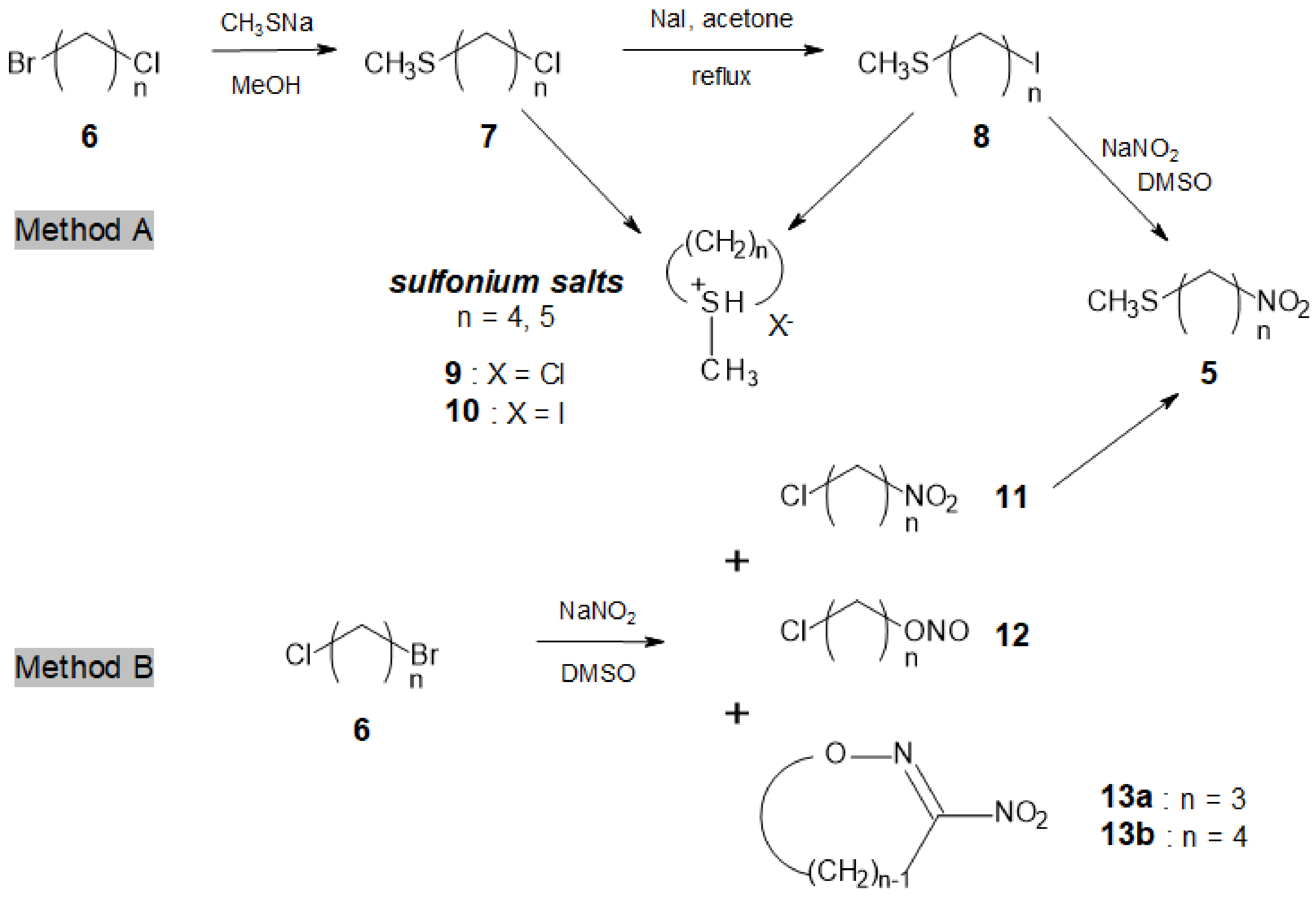
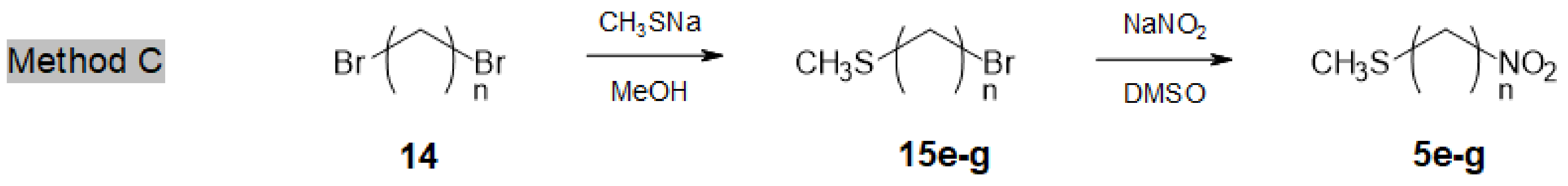3.2. Synthesis
3.2.1. General Procedure for the Synthesis of Small-Sized Chloroalkyl Methyl Sulfides 7a–d
A 2.6 M methanolic solution of MeSNa (23 mL, 1.2 equiv.) was added dropwise to a stirred solution of ω-bromochloroalkane 6 (50 mmol) in dry methanol (80 mL) at room temperature (r.t.). Stirring was continued until complete consumption of the dihalide (2–3 h, GC monitoring). The mixture was concentrated in vacuo and the resulting white slurry was taken in cold water and extracted with dichloromethane. After drying the organic phase over MgSO4, filtration and concentration in vacuo, the oily residue was purified by flash chromatography using silica gel (230–400 mesh) with hexane as eluent to afford 7a–d as light yellow oils.
ω-Chloro-1-methylsulfanylpropane
7a [
24,
40], -butane
7b [
24], -pentane
7c [
24].
6-Chloro-1-methylsulfanylhexane [98429-85-7]
7d [
41,
42].
3.2.2. General Procedure for the Synthesis of Long-Sized Bromoalkyl Methylsulfides 15e–g
Similarly, the α,ω-dibromoalkane
14 (50 mmol) dissolved in a stirred methanol (200 mL)-dichloromethane (50 mL) mixture was treated dropwise (over 30 min) by a 2.6 M methanolic solution of MeSNa (20 mL, 1.04 equiv.), while cooling at r.t. After complete consumption of the dihalide (1–2 h, TLC monitoring), the mixture was concentrated in vacuo and the resulting white slurry was taken in cold water and extracted with dichloromethane. After drying the organic phase over MgSO
4, filtration and concentration in vacuo, the oily residue was purified by flash chromatography using silica gel (230–400 mesh) with hexane as eluent to afford
15e–
g [
25].
8-Bromo-1-methylsulfanyloctane [64053-04-9] 15e [
43]. Light yellow viscous oil (57% yield).
1H-NMR (CDCl
3) δ 1.25–1.44 (m, 8H, CH
2), 1.57 (qt, 2H, H-2), 1.84 (qt, 2H, H-7), 2.08 (s, 3H, MeS), 2.48 (t, 2H,
Jvic = 7.3, H-1), 3.39 (t, 2H,
Jvic = 6.8, H-8).
13C-NMR δ 15.3 (MeS), 27.9, 28.5, 28.9 (C-2-C-6), 32.6 (C-7), 33.8 (C-8), 34.1 (C-1). ESI-MS
m/
z 240.2 [M + H]
+.
10-Bromo-1-methylsulfanyldecane 15f. Light yellow solid (55% yield). 1H-NMR (CDCl3) δ 1.22–1.47 (m, 12H, CH2), 1.58 (qt, 2H, H-2), 1.85 (qt, 2H, H-9), 2.08 (s, 3H, MeS), 2.47 (t, 2H, Jvic = 7.3, H-1), 3.39 (t, 2H, Jvic = 6.8, H-10). 13C-NMR δ 15.3 (MeS), 28.0, 28.6, 29.0, 29.2 (C-2-C-8), 32.7 (C-9), 33.8 (C-10), 34.1 (C-1). ESI-MS m/z 268.3 [M + H]+.
12-Bromo-1-methylsulfanyldodecane 15g. Light yellow solid (59% yield). 1H-NMR (CDCl3) δ 1.19–1.48 (m, 16H, CH2), 1.58 (qt, 2H, H-2), 1.84 (qt, 2H, H-11), 2.08 (s, 3H, MeS), 2.48 (t, 2H, Jvic = 7.3, H-1), 3.39 (t, 2H, Jvic = 6.8, H-12). 13C-NMR δ 15.4 (MeS), 28.0, 28.6, 28.7, 29.0, 29.1, 29.3, 29.4 (C-2-C-10), 32.7 (C-11), 33.9 (C-12), 34.2 (C-1). ESI MS m/z 296.3 [M + H]+.
3.2.3. Procedures for the Synthesis of Nitroalkyl Methylsulfides 5
Method A. Synthesis of 5a (n = 3) and 5d (n = 6)
The chloroalkyl methyl sulfide 7 (42 mmol) was refluxed in dry acetone with dry NaI (13.5 g, 90 mmol) for 2–3 days until the complete conversion of the starting material (GC monitoring). After concentration in vacuo, the resulting slurry was taken in a 0.6 M aqueous sodium thiosulfate solution, and extracted with CH2Cl2. The combined organic phases were dried over MgSO4, filtered and concentrated in vacuo to afford compound 8 as fragrant pale orange oil, which was used as such in the next step.
To a solution of dry sodium nitrite (2.0 g, 29 mmol) in DMSO (80 mL), a solution of 8 (24 mmol) in DMSO (15 mL) was added dropwise, while keeping the temperature at 20–25 °C. After 2–3 h, the reaction was quenched by adding ice-water (300 mL) and repeatedly extracted with diethyl ether. The combined organic phases were dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (petroleum ether–Et2O 4:1) to afford 5a and 5d as colourless oils.
(1-Methylsulfanyl)-3-nitropropane [182258-93-1] 5a. Colourless oil (2.27 g, 40% overall yield). 1H-NMR (CDCl3) δ 2.09 (s, 3H, MeS), 2.27 (qt, 2H, H-2), 2.58 (t, 2H, Jvic = 6.8, H-1), 4.51 (t, 2H, Jvic = 6.8, H-3). 13C-NMR δ 15.1 (MeS), 26.1 (C-2), 30.4 (C-1), 73.7 (C-3). MS IS m/z 136.2 [M + H]+.
(1-Methylsulfanyl)-6-nitrohexane 5d. Colourless oil (3.13 g, 42% overall yield). 1H-NMR (CDCl3) δ 1.32–1.48 (m, 4H, H-3, H-4), 1.54–1.63 (m, 2H, H-2), 1.94–2.04, m, 2H, H-5), 2.06 (s, 3H, MeS), 2.46 (t, 2H, Jvic = 7.3, H-1), 4.36 (t, 2H, Jvic = 7.3, H-6). 13C-NMR δ 15.2 (MeS), 25.6 (C-4), 27.0 (C-5), 27.7 (C-3), 28.4 (C-2), 33.8 (C-1), 75.5 (C-6). MS IS m/z 178.3 [M + H]+.
Method B. Synthesis of 5a–d (n = 3–6) from ω-bromochloroalkanes 6a–d
To a solution of dry sodium nitrite (1.24 g, 18 mmol) in DMSO (100 mL), a solution of ω-bromochloroalkane 6 (15 mmol) in DMSO (20 mL) was added dropwise while keeping the temperature at 20–25 °C. After 2–5 h, the reaction was quenched by adding ice-water (400 mL) and was repeatedly extracted with diethyl ether. The combined organic phases were dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by column chromatography (petroleum ether–Et2O 5:1) to afford ω-chloronitroalkanes 11 as colourless oils.
1-Chloro-3-nitropropane [16694-52-3] 11a. Colourless oil (0.8 g, 42% yield)
1H-NMR (CDCl
3) δ 2.45 (qt, 2H, H-2), 3.65 (t, 2H,
Jvic = 6.3, H-1), 4.58 (t, 2H,
Jvic = 6.3, H-3).
13C-NMR δ 29.6 (C-2), 40.7 (C-1), 72.1 (C-3) [
44].
1-Chloro-4-nitrobutane [41168-66-5] 11b. Colourless oil (1.2 g, 60% yield)
1H-NMR (CDCl
3) δ 1.83–1.92 (m, 2H, H-2), 2.13–2.23 (m, 2H, H-3), 3.58 (t, 2H,
Jvic = 6.1, H-1), 4.43 (t, 2H,
Jvic = 6.8, H-4).
13C-NMR δ 24.4 (C-2), 28.8 (C-3), 43.5 (C-1), 74.6 (C-4) [
45].
1-Chloro-5-nitropentane [1173694-33-1] 11c. Colourless oil (1.5 g, 66% yield) 1H-NMR (CDCl3) δ 1.51–1.60 (m, 2H, H-3), 1.77–1.87 (m, 2H, H-2), 1.98–2.08 (m, 2H, H-4), 3.54 (t, 2H, Jvic = 6.3, H-1), 4.39 (t, 2H, Jvic = 7.0, H-5). 13C-NMR δ 23.4 (C-3), 26.4 (C-4), 31.5 (C-2), 44.2 (C-1), 75.3 (C-5).
1-Chloro-6-nitrohexane [898543-32-3] 11d. Colourless oil (1.9 g, 76% yield)
1H-NMR (CDCl
3) δ 1.38–1.55 (m, 4H, H-3, H-4), 1.73–1.83 (m, 2H, H-2), 1.97–2.07 (m, 2H, H-5), 3.53 (t, 2H,
Jvic = 6.6, H-1), 4.38 (t, 2H,
Jvic = 7.0, H-6).
13C-NMR δ 25.4 (C-4), 25.9 (C-3), 27.0 (C-5), 31.9 (C-2), 44.6 (C-1), 75.4 (C-6) [
46].
A solution of chloronitro derivative
11 (25 mmol) in dry methanol (50 mL) was reacted with a 2.6 M methanolic solution of MeSNa (20 mL, 2.08 equiv.) under the conditions that are reported in
Table 3. After concentration in vacuo, the resulting slurry was poured into ice water (200 mL) under vigorous stirring and treated dropwise at 0 °C by a cooled solution of hydroxylamine hydrochloride (4.0 g, 57 mmol) in 20% aqueous acetic acid (17 mL) [
28]. The two-phase system obtained was extracted three times with CH
2Cl
2, and the combined organic phases were dried over MgSO
4, filtered and concentrated in vacuo. The residue was purified by column chromatography (petroleum ether–Et
2O 4:1) to afford
5a–
d as colourless oils (
5a and
5d were described above).
(1-Methylsulfanyl)-4-nitrobutane [182258-94-2] 5b. Colourless oil (1.6 g, 43% yield). 1H-NMR (CDCl3) δ 1.63–1.73 (m, 2H, H-2), 2.03–2.16 (m, 5H, MeS, H-3), 2.52 (t, 2H, Jvic = 7.0, H-1), 4.40 (t, 2H, Jvic = 7.0, H-4). 13C-NMR δ 15.1 (MeS), 25.2 (C-2), 25.9 (C-3), 33.0 (C-1), 75.0 (C-4). MS IS m/z 150.2 [M + H]+.
(1-Methylsulfanyl)-5-nitropentane [182258-95-3] 5c. Colourless oil (3.5 g, 86% yield). 1H-NMR (CDCl3) d 1.42–1.52 (m, 2H, H-3), 1.58–1.68 (m, 2H, H-2), 1.96–2.06 (m, 2H, H-4), 2.07 (s, 3H, MeS), 2.48 (t, 2H, Jvic = 7.1, H-1), 4.37 (t, 2H, Jvic = 7.1, H-5). 13C-NMR δ 15.2 (MeS), 25.0 (C-3), 26.7 (C-4), 28.00 (C-2), 33.5 (C-1), 75.3 (C-5). MS IS m/z 164.2 [M + H]+.
Method C. Synthesis of Long-Sized Nitroalkyl Methylsulfides 5e–g (n = 8, 10, 12)
To a solution of dry sodium nitrite (2.0 g, 29 mmol) in DMSO (80 mL), a solution of 15 (24 mmol) in DMSO (15 mL) was added dropwise, while keeping the temperature at 20–25 °C. After 2–3 h, the reaction was quenched by adding ice-water (300 mL) and repeatedly extracted with diethyl ether. The combined organic phases were dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by column chromatography (petroleum ether-Et2O 19:1) to afford 5e–g as colourless oils.
(1-Methylsulfanyl)-8-nitrooctane 5e. Colourless oil (3.2 g, 65% yield). 1H-NMR (CDCl3) δ 1.23–1.42 (m, 8H, H-3-6), 1.57 (qt, 2H, H-2), 1.98 (qt, 2H, H-7), 2.07 (s, 3H, MeS), 2.46 (t, 2H, Jvic = 7.3, H-1), 4.36 (t, 2H, Jvic = 7.3, H-8). 13C-NMR δ 15.3 (MeS), 25.9 (C-4), 27.1 (C-7), 28.3, 28.5, 28.6, (C-3, C-5, C-6), 28.8 (C-2), 34.0 (C-1), 75.6 (C-8). MS IS m/z 206.3 [M + H]+.
(1-Methylsulfanyl)-10-nitrodecane 5f. Colourless oil (3.8 g, 68% yield). 1H-NMR (CDCl3) δ 1.21–1.41 (m, 12H, H-3-8), 1.57 (qt, 2H, H-2), 1.99 (qt, 2H, H-9), 2.07 (s, 3H, MeS), 2.47 (t, 2H, Jvic = 7.3, H-1), 4.36 (t, 2H, Jvic = 7.3, H-10). 13C-NMR δ 15.3 (MeS), 26.0, 27.2, 28.5, 28.6, 28.9, 29.0 (C-3-9), 29.1 (C-2), 34.1 (C-1), 75.6 (C-10). MS IS m/z 234.4 [M + H]+.
(1-Methylsulfanyl)-12-nitrododecane 5g. Colourless oil (4.2 g, 67% yield). 1H-NMR (CDCl3) δ 1.21–1.42 (m, 16H, H-3-10), 1.58 (qt, 2H, H-2), 2.0 (qt, 2H, H-11), 2.09 (s, 3H, MeS), 2.48 (t, 2H, Jvic = 7.3, H-1), 4.37 (t, 2H, Jvic = 7.3, H-12). 13C-NMR δ 15.3 (MeS), 26.0, 27.2, 28.6, 28.9, 29.2 (C-3-11), 29.3 (C-2), 34.1 (C-1), 75.6 (C-12). MS IS m/z 262.4 [M + H]+.
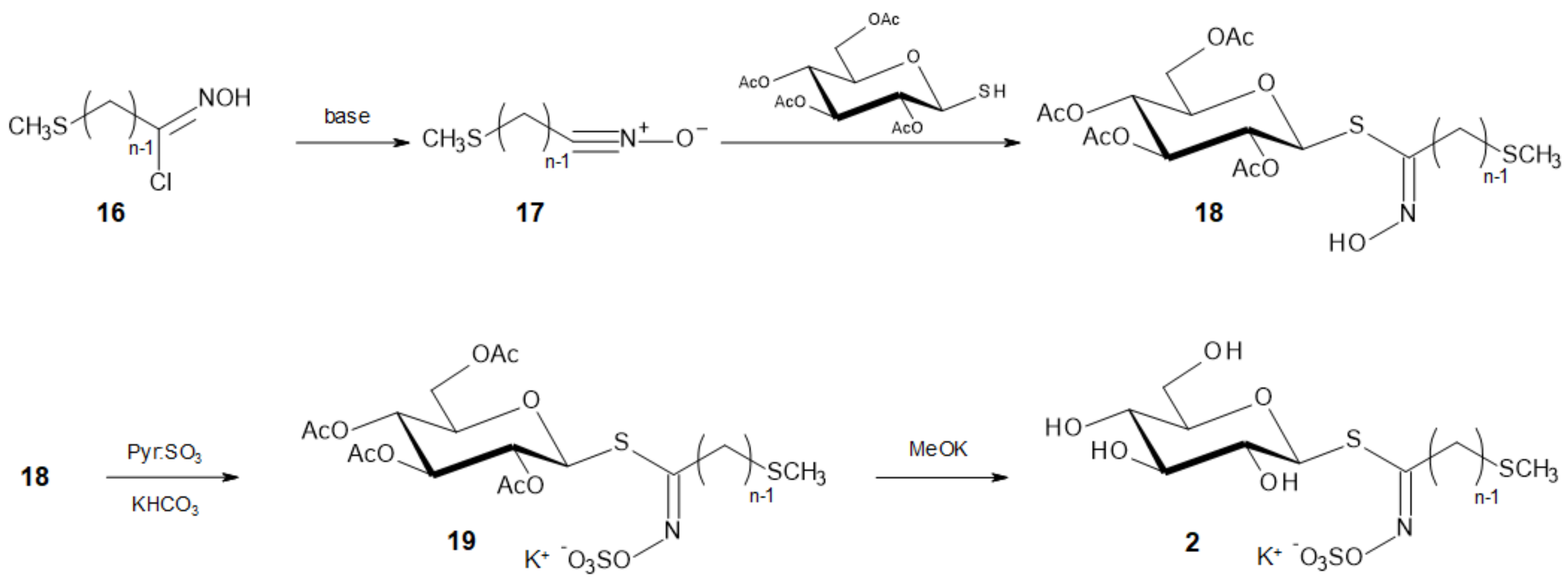
3.2.4. General Procedure for the Nitronate Chlorination and Coupling with the Thioglucose Unit
Nitronate formation. To a stirred freshly prepared solution of sodium (0.3 g) in 2-butanol (17 mL), under argon atmosphere, a solution of nitro derivative 5 (13 mmol) in anhydrous ether (20 mL) is added dropwise under exclusion of moisture. The slow addition of anhydrous ether (200 mL) while stirring caused precipitation of the nitronate as a white solid. After 10 min more stirring, the suspension was rapidly filtered off on sintered glass, and, after rinsing with anhydrous ether (20–30 mL), the nitronate cake was dried in vacuo for 30 min (preparation of nitronates can be more confortably realized in a glovebox).
Conversion of nitronate into hydroximoyl chloride. The powdered nitronate obtained was suspended in dry chloroform (40 mL), the stirred mixture was cooled at −60 °C, and a chloroform solution of freshly distilled thionyl chloride (1 mL in 5 mL) was added dropwise. After 20 min more stirring at −60°C, the reaction was quenched by pouring the mixture into ice water; the chloroform solution was separated and the aqueous phase was extracted twice with chloroform. The combined organic phases were dried over MgSO4, filtered and concentrated in vacuo to afford the raw hydroximoyl chloride as a greenish oil.
Coupling with the thioglucose unit. This oily residue was immediately dissolved in 70 mL of a dry dichloromethane–diethyl ether mixture (2:1 v/v), 2,3,4,6-tetra-O-acetyl-1-thio-β-d-glucopyranose (3.64 g, 10 mmol) was added, then dry argon was bubbled for 15 min in the solution cooled to −10 °C. A solution of triethylamine (4.2 mL, 30 mmol) in dichloromethane (10 mL) was added in one portion and the mixture was stirred during 45 min more. After washing with ice-cold 1N sulfuric acid, the organic phase was separated and the aqueous phase extracted twice with dichloromethane. The combined organic phases were dried over MgSO4, filtered and concentrated in vacuo. The syrupy residue was purified by column chromatography (petroleum ether–EtOAc 3:2) to afford the glycosyl thiohydroximates 18 as amorphous solids.
S-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl) (Z)-(3′-methylsulfanyl)propanethiohydroximate 18a. White amorphous powder (32% yield), [α]D −20 (c = 1.0, CHCl3). 1H-NMR (CDCl3) δ 2.00, 2.03, 2.05, 2.10, (4s, 12H, CH3CO), 2.14 (s, 3H, MeS), 2.78–2.81 (m, 4H, H-2′, H-3′), 3.78 (dt, 1H, J4–5 = 10.0, H-5), 4.17 (d, 2H, J5–6a = J5–6b = 3.9, H-6a, H-6b), 5.05–5.12 (m, 3H, H-1, H-2, H-4), 5.24–5.30 (m, 1H, H-3), 8.04 (brs, NOH). 13C-NMR δ 15.5 (MeS), 20.3, 20.4, 20.6 (CH3CO), 31.1 (C-2′), 32.7 (C-1′), 62.0 (C-6), 67.9 (C-4), 70.0 (C-2), 73.6 (C-3), 75.9 (C-5), 79.9 (C-1), 150.8 (C=N), 169.4, 169.5, 170.3, 170.8 (C=O). HR-ESI-MS: C18H27NO10S2: calcd. 481.1076; found 481.1061.
S-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl) (Z)-(4′-methylsulfanyl)butanethiohydroximate 18b. White amorphous powder (34% yield), [α]D −16 (c = 1.0, CHCl3). 1H-NMR (CDCl3) δ 1.90–1.95 (m, 2H, H-3′), 1.99, 2.01, 2.03, 2.06 (4s, 12H, CH3CO), 2.10 (s, 3H, MeS), 2.52–2.67 (m, 4H, H-2′, H-4′), 3.82 (ddd, 1H, J4–5 = 9.9, H-5), 4.13 (dd, 1H, J5–6b = 2.4, Jgem = 11.8, H-6b), 4.20 (dd, 1H, J5–6a = 5.0, H-6a), 5.00–5.12 (m, 3H, H-1, H-2, H-4), 5.24 (t, 1H, J3–4 = 9.0, H-3), 8.80 (brs, NOH). 13C-NMR δ 15.4 (MeS), 20.4, 20.5, 20.6 (CH3CO), 26.1 (C-3′), 30.9 (C-4′), 33.4 (C-2′), 62.0 (C-6), 67.9 (C-4), 70.1 (C-2), 73.8 (C-3), 75.8 (C-5), 79.8 (C-1), 151.7 (C=N), 169.3, 169.5, 170.4, 170.8 (C=O). HR-ESI-MS: C19H29NO10S2: calcd. 495.1233; found 495.1230.
S-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl) (Z)-(5′-methylsulfanyl)pentanethiohydroximate 18c. White amorphous powder (40% yield), [α]D −17 (c = 1.0, CHCl3). 1H-NMR (CDCl3) δ 1.60–1.85 (m, 4H, H-3′, H-4′), 2.02, 2.05, 2.07, 2.09 (4s, 12H, CH3CO), 2.11 (s, 3H, MeS), 2.45–2.55 (m, 4H, H-2′, H-5′), 3.79 (ddd, 1H, J4–5 = 9.7, H-5), 4.14 (dd, 1H, J5–6b = 2.5, Jgem = 12.6, H-6b), 4.22 (dd, 1H, J5–6a = 5.4, H-6a), 5.05–5.15 (m, 3H, H-1, H-2, H-4), 5.25 (dd, 1H, J3–4 = 9.3, H-3), 8.20 (brs, NOH). 13C-NMR δ 15.3 (MeS), 20.4, 20.5 (CH3CO), 25.8 (C-4′), 28.0 (C-3′), 31.9 (C-5′), 33.5 (C-2′), 62.1 (C-6), 68.0 (C-4), 70.1 (C-2), 73.7 (C-3), 75.8 (C-5), 79.8 (C-1), 152.0 (C=N), 169.3, 169.5, 170.3, 170.8 (C=O). HR-ESI-MS: C20H31NO10S2: calcd. 509.1389; found 509.1378.
S-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl) (Z)-(6′-methylsulfanyl)hexanethiohydroximate 18d. White amorphous powder (48% yield), [α]D −11 (c = 1.0, CHCl3). 1H-NMR (CDCl3) δ 1.40–1.51 (m, 2H, H-4′), 1.58–1.72 (m, 4H, H-3′, H-5′), 2.01, 2.02, 2.04, 2.08 (4s, 12H, CH3CO), 2.10 (s, 3H, MeS), 2.50 (t, 4H, Jvic = 7.1, H-2′, H-6′), 3.75 (ddd, 1H, J4–5 = 10.1, H-5), 4.11 (dd, 1H, J5–6b = 2.4, Jgem = 12.3, H-6b), 4.20 (dd, 1H, J5–6a = 5.6, H-6a), 5.04–5.12 (m, 3H, H-1, H-2, H-4), 5.27 (dd, 1H, J3–4 = 9.3, H-3), 8.20 (brs, NOH). 13C-NMR δ 15.3 (MeS), 20.4, 20.5 (CH3CO), 26.4 (C-4′), 28.0 (C-5′), 28.5 (C-3′), 32.3 (C-6′), 33.8 (C-2′), 62.1 (C-6), 68.0 (C-4), 70.0 (C-2), 73.7 (C-3), 75.9 (C-5), 79.8 (C-1), 152.1 (C=N), 169.3, 169.5, 170.5, 170.7 (C=O). HR-ESI-MS: C21H33NO10S2: calcd. 523.1546; found 523.1541.
S-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl) (Z)-(8′-methylsulfanyl)octanethiohydroximate 18e. White amorphous powder (47% yield), [α]D −11 (c = 1.0, CHCl3). 1H-NMR (CDCl3) δ 1.32–1.40 (m, 6H, H-4′, H-5′, H-6′), 1.47–1.64 (m, 4H, H-3′, H-7′), 1.99, 2.01, 2.02, 2.05 (4s, 12H, CH3CO), 2.07 (s, 3H, MeS), 2.41–2.46 (m, 4H, H-2′, H-8′), 3.73 (ddd, 1H, J4–5 = 10.0, H-5), 4.09 (dd, 1H, J5–6b = 2.1, Jgem = 12.3, H-6b), 4.18 (dd, 1H, J5–6a = 5.4, H-6a), 5.02–5.10 (m, 3H, H-1, H-2, H-4), 5.25 (dd, 1H, J3–4 = 9.4, H-3), 8.83 (brs, NOH). 13C-NMR δ 15.3 (MeS), 20.4, 20.5 (CH3CO), 26.8, 28.5, 28.7 (C-4′, C-5′, C-6′), 28.8 (C-7′), 28.9 (C-3′), 32.3 (C-8′), 34.5 (C-2′), 62.1 (C-6), 68.0 (C-4), 70.0 (C-2), 73.7 (C-3), 75.9 (C-5), 79.8 (C-1), 152.2 (C=N), 169.3, 169.5, 170.4, 170.7 (C=O). HR-ESI-MS: C23H37NO10S2: calcd. 551.1859; found 551.1851.
S-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl) (Z)-(10′-methylsulfanyl)decanethiohydroximate 18f. White amorphous powder (51% yield), [α]D −12 (c = 1.0, CHCl3). 1H-NMR (CDCl3) δ 1.28–1.34 (m, 10H, H-4′ to H-8′), 1.51–1.63 (m, 4H, H-3′, H-9′), 1.98, 2.01, 2.02, 2.04 (4s, 12H, CH3CO), 2.06 (s, 3H, MeS), 2.38–2.47 (m, 4H, H-2′, H-10′),3.71 (ddd, 1H, J4–5 = 10.0, H-5), 4.08 (dd, 1H, J5–6b = 2.4, Jgem = 12.1, H-6b), 4.19 (dd, 1H, J5–6a = 5.2, H-6a), 5.00–5.09 (m, 3H, H-1, H-2, H-4), 5.22 (dd, 1H, J3–4 = 9.2, H-3), 8.86 (brs, NOH). 13C-NMR δ 15.3 (MeS), 20.4, 20.5 (CH3CO), 26.9, 28.6, 28.9, (C-4′ to C-8′), 29.0 (C-9′), 29.2 (C-3′), 32.4 (C-10′), 34.1 (C-2′), 62.1 (C-6), 68.0 (C-4), 70.0 (C-2), 73.7 (C-3), 75.9 (C-5), 79.8 (C-1), 152.3 (C=N), 169.3, 169.5, 170.4, 170.7 (C=O). HR-ESI-MS: C25H41NO10S2: calcd. 579.2172; found 579.2163.
S-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl) (Z)-(12′-methylsulfanyl)dodecanethiohydroximate 18g. White amorphous powder (56% yield), [α]D −12 (c = 1.0, CHCl3). 1H-NMR (CDCl3) δ 1.31–1.38 (m, 14H, H-4′ to H-10′), 1.53–1.66 (m, 4H, H-3′, H-11′), 1.98, 2.01, 2.02, 2.05 (4s, 12H, CH3CO), 2.07 (s, 3H, MeS), 2.40–2.45 (m, 4H, H-2′, H-12′), 3.72 (ddd, 1H, J4–5 = 9.8, H-5), 4.09 (dd, 1H, J5–6b = 2.5, Jgem = 12.5, H-6b), 4.19 (dd, 1H, J5–6a = 5.5, H-6a), 5.01–5.12 (m, 3H, H-1, H-2, H-4), 5.24 (dd, 1H, J3–4 = 9.5, H-3), 8.88 (brs, NOH). 13C-NMR δ 15.3 (MeS), 20.4, 20.5 (CH3CO), 26.7, 28.9, 29.0 (C-4′ to C-10′), 29.1 (C-11′), 29.2 (C-3′), 32.4 (C-12′), 34.3 (C-2′), 62.1 (C-6), 67.9 (C-4), 70.0 (C-2), 73.6 (C-3), 75.8 (C-5), 79.8 (C-1), 152.3 (C=N), 169.2, 169.5, 170.3, 170.6 (C=O). HR-ESI-MS: C27H45NO10S2: calcd. 607.2485; found 607.2478.
3.2.5. General Procedure for NO-Sulfation of the Glucosyl Thiohydroximates 18
Sulfur trioxide pyridine complex (400 mg, 5 eq.) was added to a solution of compound 18 (0.5 mmol) in dimethylformamide (7 mL). After 24 h stirring at rt, the reaction mixture was treated with a 0.2 M aqueous solution of KHCO3 (12 mL) then the solvents were evaporated in vacuo. Chromatographic purification (CH2Cl2/MeOH 17:3) provided compounds 19.
Per-O-acetylated 2-methylsulfanylethyl glucosinolate 19a. White amorphous powder (61% yield), [α]D −20 (c = 0.9, MeOH). 1H-NMR (DMSO-d6) δ 1.96, 1.99, 2.01, 2.03 (4s, 12H, CH3CO), 2.23 (s, 3H, MeS), 2.46–2.53 (m, 4H, H-1′, H-2′), 4.03–4.15 (m, 3H, H-5, H-6a, H-6b), 4.88 (t, 1H, J2–3 = 9.4, H-2), 4.94 (t, 1H, J4–5 = 8.6, H-4), 5.39 (dd, 1H, J3–4 = 9.6, H-3), 5.56 (d, 1H, J1–2 = 10.1, H-1). 13C-NMR δ 14.6 (MeS), 20.2, 20.3, 20.5 (CH3CO), 26.5 (C-2′), 32.9 (C-1′), 62.2 (C-6), 68.1 (C-4), 69.8 (C-2), 72.9 (C-3), 74.0 (C-5), 78.3 (C-1), 153.2 (C=N), 169.4, 169.7, 170.0, 170.3 (C=O). HR-ESI-MS: C18H26NO13S3: calcd. 560.0566; found 560.0571.
Per-O-acetylated 3-methylsulfanylpropyl glucosinolate 19b. White amorphous powder (68% yield), [α]D −22 (c = 1.1, MeOH). 1H-NMR (DMSO-d6) δ 1.79–1.90 (m, 2H, H-2′), 1.94, 1.97, 1.99, 2.00 (4s, 12H, CH3CO), 2.05 (s, 3H, MeS), 2.53 (t, 2H, Jvic = 7.3, H-3′), 2.63 (t, 2H, Jvic = 7.3, H-1′), 4.00–4.18 (m, 3H, H-5, H-6a, H-6b), 4.86 (t, 1H, J2–3 = 9.0, H-2), 4.89 (t, 1H, J4–5 = 8.8, H-4), 5.43 (dd, 1H, J3–4 = 9.3, H-3), 5.50 (d, 1H, J1–2 = 10.2, H-1). 13C-NMR δ 14.7 (MeS), 20.2, 20.3, 20.5 (CH3CO), 26.4 (C-3′), 30.3, 32.5 (C-1′, C-2′), 62.1 (C-6), 68.0 (C-4), 69.6 (C-2), 72.8 (C-3), 74.4 (C-5), 78.2 (C-1), 153.5 (C=N), 169.4, 169.6, 169.9, 170.3 (C=O). HR-ESI-MS: C19H28NO13S3: calcd. 574.0723; found 574.0730.
Per-O-acetylated 4-methylsulfanylbutyl glucosinolate 19c. White amorphous powder (86% yield), [α]D −18 (c = 1.0, MeOH). 1H-NMR (DMSO-d6) δ 1.52–1.68 (m, 4H, H-2′, H-3′), 1.94, 1.97, 1.99, 2.00 (4s, 12H, CH3CO), 2.02 (s, 3H, MeS), 2.48–2.59 (m, 4H, H-1′, H-4′), 4.00–4.15 (m, 3H, H-5, H-6a, H-6b), 4.86 (t, 1H, J2–3 = 9.5, H-2), 4.91 (t, 1H, J4–5 = 8.5, H-4), 5.43 (dd, 1H, J3–4 = 9.5, H-3), 5.50 (d, 1H, J1–2 = 10.2, H-1). 13C-NMR δ 14.5 (MeS), 20.2, 20.3, 20.5 (CH3CO), 25.7 (C-4′), 27.9, 31.0, 32.8 (C-1′, C-2′, C-3′), 62.2 (C-6), 68.1 (C-4), 69.6 (C-2), 72.8 (C-3), 74.4 (C-5), 78.2 (C-1), 154.0 (C=N), 169.4, 169.6, 169.9, 170.3 (C=O). HR-ESI-MS: C20H30NO13S3: calcd. 588.0879; found 588.0887.
Per-O-acetylated 5-methylsulfanylpentyl glucosinolate 19d. White amorphous powder (84% yield), [α]D −15 (c = 1.1, MeOH). 1H-NMR (DMSO-d6) δ 1.38–1.68 (m, 6H, H-2′, H-3′, H-4′), 1.94, 1.98, 1.99, 2.00 (4s, 12H, CH3CO), 2.02 (s, 3H, MeS), 2.43–2.57 (m, 4H, H-1′, H-5′), 3.98–4.15 (m, 3H, H-5, H-6a, H-6b), 4.86 (t, 1H, J2–3 = 9.8, H-2), 4.91 (t, 1H, J4–5 = 8.8, H-4), 5.45 (dd, 1H, J3–4 = 9.7, H-3), 5.51 (d, 1H, J1–2 = 10.2, H-1). 13C-NMR δ 15.6 (MeS), 20.2, 20.4, 20.5 (CH3CO), 26.4 (C-5′), 27.8, 28.2, 31.4, 33.2 (C-1′ to C-4′), 62.2 (C-6), 68.2 (C-4), 69.6 (C-2), 72.8 (C-3), 74.4 (C-5), 78.2 (C-1), 154.1 (C=N), 169.4, 169.6, 169.9, 170.3 (C=O). HR-ESI-MS: C21H32NO13S3: calcd. 602.1036; found 602.1041.
Per-O-acetylated 7-methylsulfanylheptyl glucosinolate 19e. White amorphous powder (83% yield), [α]D −17 (c = 1.0, MeOH). 1H-NMR (DMSO-d6) δ 1.24–1.39 (m, 6H, H-3′, H-4′, H-5′), 1.44–1.65 (m, 4H, H-2′, H-6′), 1.94, 1.97, 1.99, 2.01 (4s, 12H, CH3CO), 2.01 (s, 3H, MeS), 2.40–2.54 (m, 4H, H-1′, H-7′), 3.98–4.15 (m, 3H, H-5, H-6a, H-6b), 4.85 (t, 1H, J2–3 = 9.2, H-2), 4.91 (t, 1H, J4–5 = 8.8, H-4), 5.46 (dd, 1H, J3–4 = 9.4, H-3), 5.49 (d, 1H, J1–2 = 10.3, H-1). 13C-NMR δ 14.7 (MeS), 20.2, 20.3, 20.4 (CH3CO), 26.8 (C-7′), 28.2, 28.3, 28.6, 31.4, 33.2 (C-1′ to C-6′), 62.2 (C-6), 68.2 (C-4), 69.6 (C-2), 72.8 (C-3), 74.4 (C-5), 78.2 (C-1), 154.1 (C=N), 169.4, 169.6, 169.9, 170.2 (C=O). HR-ESI-MS: C23H36NO13S3: calcd. 630.1049; found 630.1060.
Per-O-acetylated 9-methylsulfanylnonyl glucosinolate 19f. White amorphous powder (85% yield), [α]D −16 (c = 1.0, MeOH). 1H-NMR (DMSO-d6) δ 1.21–1.40 (m, 10H, H-3′ to H-7′), 1.44–1.59 (m, 4H, H-2′, H-8′), 1.95, 1.97, 1.98, 2.00 (4s, 12H, CH3CO), 2.01 (s, 3H, MeS), 2.40–2.54 (m, 4H, H-1′, H-9′), 3.98–4.14 (m, 3H, H-5, H-6a, H-6b), 4.84 (t, 1H, J2–3 = 9.7, H-2), 4.90 (t, 1H, J4–5 = 8.5, H-4), 5.46 (dd, 1H, J3–4 = 9.7, H-3), 5.50 (d, 1H, J1–2 = 10.0, H-1). 13C-NMR δ 15.1 (MeS), 20.2, 20.3, 20.4 (CH3CO), 26.9 (C-9′), 28.2, 28.4, 28.5, 28.8, 29.1, 31.6, 34.3 (C-1′ to C-8′), 62.2 (C-6), 68.4 (C-4), 70.0 (C-2), 73.1 (C-3), 74.7 (C-5), 78.7 (C-1), 154.5 (C=N), 169.3, 169.5, 170.0, 170.3 (C=O). HR-ESI-MS: C25H40NO13S3: calcd. 658.1662; found 658.1665.
Per-O-acetylated 11-methylsulfanylundecyl glucosinolate 19g. White amorphous powder (84% yield), [α]D −16 (c = 1.0, MeOH). 1H-NMR (DMSO-d6) δ 1.24–1.40 (m, 14H, H-3′ to H-9′), 1.42–1.63 (m, 4H, H-2′, H-10′), 1.96, 1.98, 2.00, 2.02 (4s, 12H, CH3CO), 2.01 (s, 3H, MeS), 2.47–2.56 (m, 4H, H-1′, H-11′), 3.93–4.13 (m, 3H, H-5, H-6a, H-6b), 4.86 (t, 1H, J2–3 = 9.6, H-2), 4.93 (t, 1H, J4–5 = 8.8, H-4), 5.47 (dd, 1H, J3–4 = 9.5, H-3), 5.51 (d, 1H, J1–2 = 10.0, H-1). 13C-NMR δ 14.71 (MeS), 20.2, 20.3, 20.4 (CH3CO), 26.5 (C-11′), 28.8, 29.0, 29.3, 29.5, 29.9, 31.5, 32.4, 34.8 (C-1′ to C-10′), 62.1 (C-6), 68.4 (C-4), 69.8 (C-2), 73.2 (C-3), 75.1 (C-5), 78.3 (C-1), 154.5 (C=N), 169.4, 169.6, 170.0, 170.3 (C=O). HR-ESI-MS: C27H44NO13S3: calcd. 686.1975; found 686.1987.
3.2.6. General Procedure for Deprotection of the Glucopyranosyl Moiety
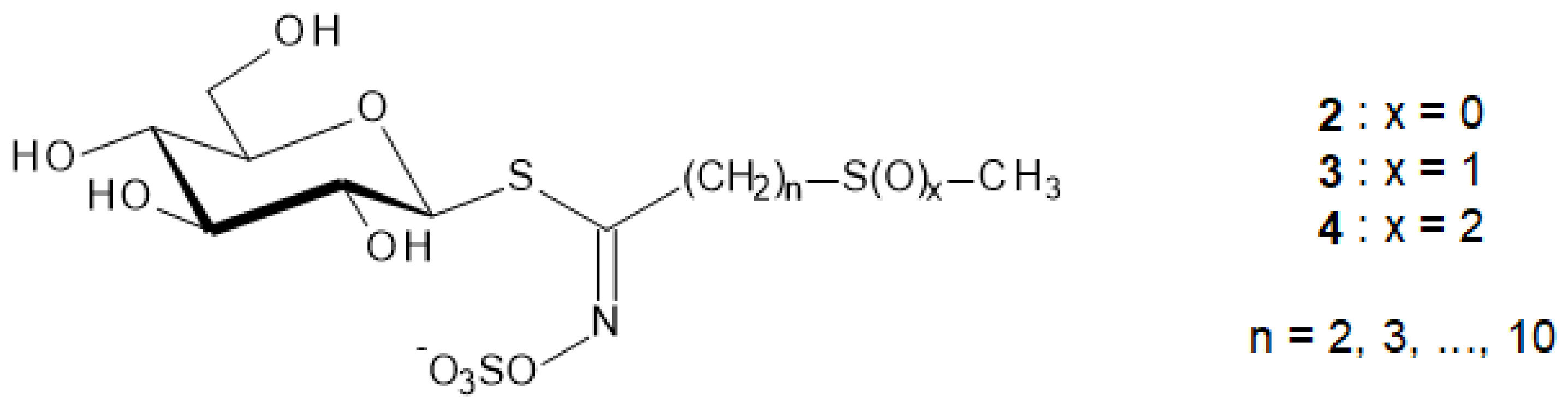
To a suspension of peracetylated glucosinolate 19 (0.1 mmol) in dry methanol (5 mL) under argon, a freshly prepared 1 M solution of potassium methoxide was added dropwise until the pH reached 9. After 4 h standing at rt, the reaction mixture was neutralized by the addition of Dowex H+ resin. After filtration and evaporation in vacuo, the resulting crude was purified by C-18 silica gel column chromatography (eluent: water) and freeze-drying to provide glucosinolates 2.
2-Methylsulfanylethyl glucosinolate [27303-30-6] 2a. White amorphous powder (80% yield), [α]D −27 (c = 0.95, H2O). 1H-NMR (D2O) δ 2.19 (s, 3H, MeS), 2.92–2.98 (m, 2H, H-2′), 3.04–3.10 (m, 2H, H-1′), 3.44–3.53 (m, 2H, H-2, H-4), 3.58–3.69 (m, 2H, H-3, H-5), 3.76 (dd, 1H, J5–6b = 5.8, Jgem = 13.0, H-6b), 3.95 (dd, 1H, J5–6a = 2.6, H-6a), 5.09 (d, 1H, J1–2 = 10.1, H-1). 13C-NMR δ 15.3 (MeS), 30.5 (C-2′), 33.0 (C-1′), 61.5 (C-6), 70.2 (C-4), 73.5 (C-2), 78.3 (C-3), 81.5 (C-5), 83.1 (C-1), 164.9 (C=N). HR-ESI-MS: C10H18NO9S3: calcd. 392.0144; found 392.0133.
3-Methylsulfanylpropyl glucosinolate [26888-03-9] 2b. Glucoibervirin. White amorphous powder (84% yield), [α]D −21 (c = 1.05, H2O). 1H-NMR (D2O) δ 2.05 (qt, 2H, H-2′), 2.16 (s, 3H, MeS), 2.67 (t, 2H, Jvic = 7.5, H-3′), 2.63 (t, 2H, Jvic = 7.5, H-1′), 3.46–3.52 (m, 2H, H-2, H-4), 3.55–3.66 (m, 2H, H-3, H-5), 3.73 (dd, 1H, J5–6b = 5.8, Jgem = 13.2, H-6b), 3.93 (dd, 1H, J5–6a = 2.7, H-6a), 5.09 (d, 1H, J1–2 = 10.1, H-1). 13C-NMR δ 15.3 (MeS), 26.7 (C-2′), 30.9 (C-3′), 34.1 (C-1′), 61.4 (C-6), 70.7 (C-4), 73.2 (C-2), 78.2 (C-3), 82.5 (C-5), 83.8 (C-1), 165.1 (C=N). HR-ESI-MS: C11H20NO9S3: calcd. 406.0300; found 406.0291.
4-Methylsulfanylbutyl glucosinolate [21973-56-8] 2c.
Glucoerucin. White amorphous powder (90% yield), [α]
D −20 (
c = 1.00, H
2O).
1H-NMR (D
2O) δ 1.67–1.89 (m, 4H, CH
2), 2.12 (s, 3H, MeS), 2.61 (t, 2H,
Jvic = 7.5, H-4′), 2.76 (t, 2H,
Jvic = 7.5, H-1′), 3.42–3.51 (m, 2H, H-2, H-4), 3.57–3.63 (m, 2H, H-3, H-5), 3.72 (dd, 1H,
J5–6b = 5.8,
Jgem = 13.1, H-6b), 3.91 (dd, 1H,
J5–6a = 2.7, H-6a), 5.06 (d, 1H,
J1–2 = 10.1, H-1).
13C-NMR δ 15.2 (MeS), 25.7 (C-2′), 28.2 (C-3′), 31.6 (C-4′), 33.0 (C-1′), 62.2 (C-6), 71.2 (C-4), 73.5 (C-2), 78.7 (C-3), 81.8 (C-5), 83.0 (C-1), 165.4 (C=N) [
47]. HR-ESI-MS: C
12H
22NO
9S
3: calcd. 420.0457; found 420.0453.
5-Methylsulfanylpentyl glucosinolate [29611-01-6] 2d.
Glucoberteroin. White amorphous powder (90% yield), [α]
D −21 (
c = 0.95, H
2O).
1H-NMR (D
2O) δ 1.44 (qt, 2H,
Jvic = 7.4, H-3′), 1.61 (qt, 2H,
Jvic = 7.3, H-4′) 1.70 (qt, 2H,
Jvic = 7.3, H-2′), 2.06 (s, 3H, MeS), 2.53 (t, 2H,
Jvic = 7.3, H-5′), 2.68 (t, 2H,
Jvic = 7.3, H-1′), 3.38–3.45 (m, 2H, H-2, H-4), 3.50–3.56 (m, 2H, H-3, H-5), 3.68 (dd, 1H,
J5–6b = 5.8,
Jgem = 13.1, H-6b), 3.85 (dd, 1H,
J5–6a = 2.6, H-6a), 5.00 (d, 1H,
J1–2 = 10.0, H-1).
13C-NMR δ 15.1 (
MeS), 27.5 (C-3′), 28.5 (C-2′), 29.0 (C-4′), 33.0 (C-5′), 34.2 (C-1′), 61.8 (C-6), 70.3 (C-4), 73.3 (C-2), 78.4 (C-3), 81.4 (C-5), 83.0 (C-1), 164.5 (C=N) [
48].HR-ESI-MS: C
13H
24NO
9S
3: calcd. 434.0613; found 434.0610.
7-Methylsulfanylheptyl glucosinolate [80667-67-0] 2e. White amorphous powder (90% yield), [α]
D −22 (
c = 1.00, H
2O).
1H-NMR (D
2O) δ 1.24–1.39 (m, 6H, CH
2), 1.44–1.65 (m, 4H, CH
2), 2.01 (s, 3H, MeS), 2.38–2.54 (m, 4H, CH
2), 3.45–3.52 (m, 2H, H-2, H-4), 3.55–3.63 (m, 2H, H-3, H-5), 3.68 (dd, 1H,
J5–6b = 5.7,
Jgem = 12.8, H-6b), 3.89 (dd, 1H,
J5–6a = 2.6, H-6a), 5.06 (d, 1H,
J1–2 = 10.0, H-1).
13C-NMR δ 15.1(MeS), 27.8, 28.7, 28.9 (C-3′-C-5′), 29.0 (C-2′), 29.2 (C-6′), 33.0 (C-1′), 34.3 (C-7′), 61.6 (C-6), 70.1 (C-4), 73.0 (C-2), 78.2 (C-3), 81.2 (C-5), 82.9 (C-1), 165.4 (C=N). HR-ESI
--MS: C
15H
28NO
9S
3: calcd. 462.0926; found 462.0932 [
48]. HR-ESI-MS: C
15H
28NO
9S
3: calcd. 462.0926; found 462.0919.
9-Methylsulfanylnonyl glucosinolate [81149-01-1] 2f. White amorphous powder (93% yield), [α]D −19 (c = 0.84, H2O). 1H-NMR (D2O) δ 1.21–1.40 (m, 10H, CH2), 1.44–1.59 (m, 4H, CH2), 2.00 (s, 3H, MeS), 2.43–2.58 (m, 4H, (CH2), 3.40–3.51 (m, 2H, H-2, H-4), 3.57–3.62 (m, 2H, H-3, H-5), 3.71 (dd, 1H, J5–6b = 5.7, Jgem = 13.0, H-6b), 3.91 (dd, 1H, J5–6a = 2.7, H-6a), 5.06 (d, 1H, J1–2 = 10.1, H-1). 13C-NMR δ 15.4 (MeS), 26.9, 28.2, 28.4, 28.6 (C-3′-C-7′), 28.9 (C-2′), 29.2 (C-8′), 31.9 (C-1′), 34.6 (C-9′), 61.4 (C-6), 70.5 (C-4), 73.4 (C-2), 78.3 (C-3), 82.2 (C-5), 83.5 (C-1), 164.8 (C=N). HR-ESI-MS: C17H32NO9S3: calcd. 490.1239; found 490.1231.
11-Methylsulfanylundecyl glucosinolate 2g. Colourless gum (91% yield), [α]D −21 (c = 0.92, H2O). 1H-NMR δ (D2O) 1.26–1.44 (m, 10H, CH2), 1.42–1.63 (m, 4H, (CH2), 2.02 (s, 3H, MeS), 2.44–2.56 (m, 4H, (CH2), 3.39–3.50 (m, 2H, H-2, H-4), 3.54–3.61 (m, 2H, H-3, H-5), 3.74 (dd, 1H, J5–6b = 5.8, Jgem = 13.0, H-6b), 3.93 (dd, 1H, J5–6a = 2.6,, H-6a), 5.06 (d, 1H, , J1–2 = 10.1, H-1). 13C-NMR δ 15.2 (MeS), 26.5, 28.9, 29.0, 29.5 (C3′-C-9′), 29.7 (C-2′), 30.0 (C-10′), 32.2 (C-1′), 34.8 (C-11′), 61.6 (C-6), 70.2 (C-4), 73.1 (C-2), 78.7 (C-3), 81.3 (C-5), 83.0 (C-1), 165.4 (C=N). HR-ESI-MS: C19H36NO9S3: calcd. 518.1552; found 518.1547.