The Stability of Medicinal Plant microRNAs in the Herb Preparation Process
Abstract
:1. Introduction
2. Results
2.1. Total RNA Isolation from Raw Herb Materials and Herb Preparations
2.2. The Stability of miRNA during the Herb Preparation Process
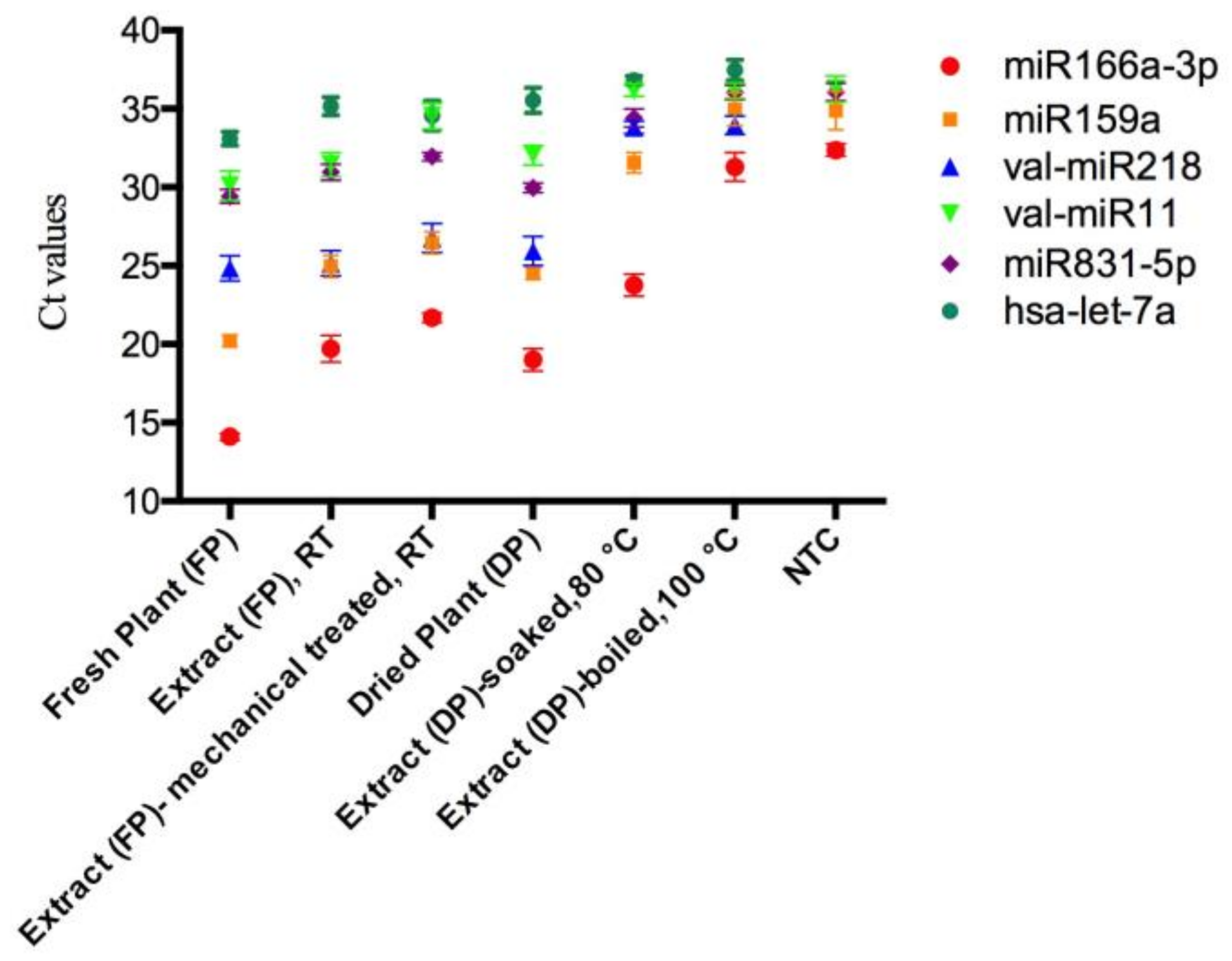
2.3. The Stability of Synthetic miRNAs
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Preparations of Mistletoe Extracts
4.3. RNA Isolation
4.4. qRT-PCR
4.5. Treatment of Synthetic miRNAs
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous Plant MIR168a Specifically Targets Mammalian LDLRAP1: Evidence of Cross-Kingdom Regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, X.; Liu, J.; Dong, L.; Chen, Q.; Liu, J.; Kong, H.; Zhang, Q.; Qi, X.; Hou, D.; et al. Honeysuckle-Encoded Atypical microRNA2911 Directly Targets Influenza A Viruses. Cell Res. 2014, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.R.; Fong, M.Y.; Somlo, G.; Wu, J.; Swiderski, P.; Wu, X.; Wang, S.E. Cross-Kingdom Inhibition of Breast Cancer Growth by Plant miR159. Cell Res. 2016, 26, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Weng, A.; Melzig, M. MicroRNAs as New Bioactive Components in Medicinal Plants. Planta Med. 2016, 82, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Philip, A.; Ferro, V.A.; Tate, R.J. Determination of the Potential Bioavailability of Plant microRNAs Using a Simulated Human Digestion Process. Mol. Nutr. Food Res. 2015, 59, 1962–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Adolf, J.; Melzig, M.F. Identification of Viscum Album L. miRNAs and Prediction of Their Medicinal Values. PLoS ONE 2017, 12, e0187776. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, V.; Pierson, S.; Kaoma, T.; Bernardin, F.; Berchem, G. Assessing Cellular and Circulating miRNA Recovery: The Impact of the RNA Isolation Method and the Quantity of Input Material. Sci. Rep. 2016, 6, 19529. [Google Scholar] [CrossRef] [PubMed]
- Mlotshwa, S.; Pruss, G.J.; MacArthur, J.L.; Endres, M.W.; Davis, C.; Hofseth, L.J.; Peña, M.M.; Vance, V. A Novel Chemopreventive Strategy Based on Therapeutic microRNAs Produced in Plants. Cell Res. 2015, 25, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Horneber, M.A.; Bueschel, G.; Huber, R.; Linde, K.; Rostock, M. Mistletoe Therapy in Oncology. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef] [PubMed]
- PDQ Integrative, Alternative, and Complementary Therapies Editorial Board. Mistletoe Extracts (PDQ®): Health Professional Version; National Cancer Institute (US): Rockville, MD, USA, 2002. [Google Scholar]
- Liang, H.; Zhang, S.; Fu, Z.; Wang, Y.; Wang, N.; Liu, Y.; Zhao, C.; Wu, J.; Hu, Y.; Zhang, J.; et al. Effective Detection and Quantification of Dietetically Absorbed Plant microRNAs in Human Plasma. J. Nutr. Biochem. 2015, 26, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-Time Quantification of microRNAs by Stem-Loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef] [PubMed]
- Galli, R.; Paone, A.; Fabbri, M.; Zanesi, N.; Calore, F.; Cascione, L.; Acunzo, M.; Stoppacciaro, A.; Tubaro, A.; Lovat, F.; et al. Toll-like Receptor 3 (TLR3) Activation Induces microRNA-Dependent Reexpression of Functional RARβ and Tumor Regression. Proc. Natl. Acad. Sci. USA 2013, 110, 9812–9817. [Google Scholar] [CrossRef] [PubMed]
- Ibberson, D.; Benes, V.; Muckenthaler, M.U.; Castoldi, M. RNA Degradation Compromises the Reliability of microRNA Expression Profiling. BMC Biotechnol. 2009, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yang, Z.; Yu, B.; Liu, J.; Chen, X. Methylation Protects miRNAs and siRNAs from a 3’-end Uridylation Activity in Arabidopsis. Curr. Biol. 2005, 15, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Basak, A.; Dasgupta, S. Synthesis and Ribonuclease A Inhibition Activity of Resorcinol and Phloroglucinol Derivatives of Catechin and Epicatechin: Importance of Hydroxyl Groups. Bioorg. Med. Chem. 2010, 18, 6538–6546. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.S.; Maiti, T.K.; Debnath, J.; Dasgupta, S. Inhibition of Ribonuclease A by Polyphenols Present in Green Tea. Proteins 2007, 69, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Tai, B.H.; Nhut, N.D.; Nhiem, N.X.; Tung, N.H.; Quang, T.H.; Thuy Luyen, B.T.; Huong, T.T.; Wilson, J.; Beutler, J.A.; Ban, N.K.; et al. Evaluation of the RNase H Inhibitory Properties of Vietnamese Medicinal Plant Extracts and Natural Compounds. Pharm. Biol. 2011, 49, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Diederichs, S. Argonaute Proteins Regulate microRNA Stability: Increased microRNA Abundance by Argonaute Proteins Is due to microRNA Stabilization. RNA Biol. 2011, 8, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Mo, B.; Chen, X. Mechanisms That Impact microRNA Stability in Plants. RNA Biol. 2012, 9, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, C.; Clayton, A.; Newbury, L.; Corish, P.; Jenkins, R.; Phillips, A.; Fraser, D.; Bowen, T. Stabilization of Urinary MicroRNAs by Association with Exosomes and Argonaute 2 Protein. Non-Coding RNA 2015, 1, 151–166. [Google Scholar] [CrossRef]
- Accerbi, M.; Schmidt, S.A.; De Paoli, E.; Park, S.; Jeong, D.; Green, P.J. Methods for Isolation of Total RNA to Recover miRNAs and Other Small RNAs from Diverse Species. Plant MicroRNAs 2010, 592, 31–50. [Google Scholar]
Sample Availability: Not available. |
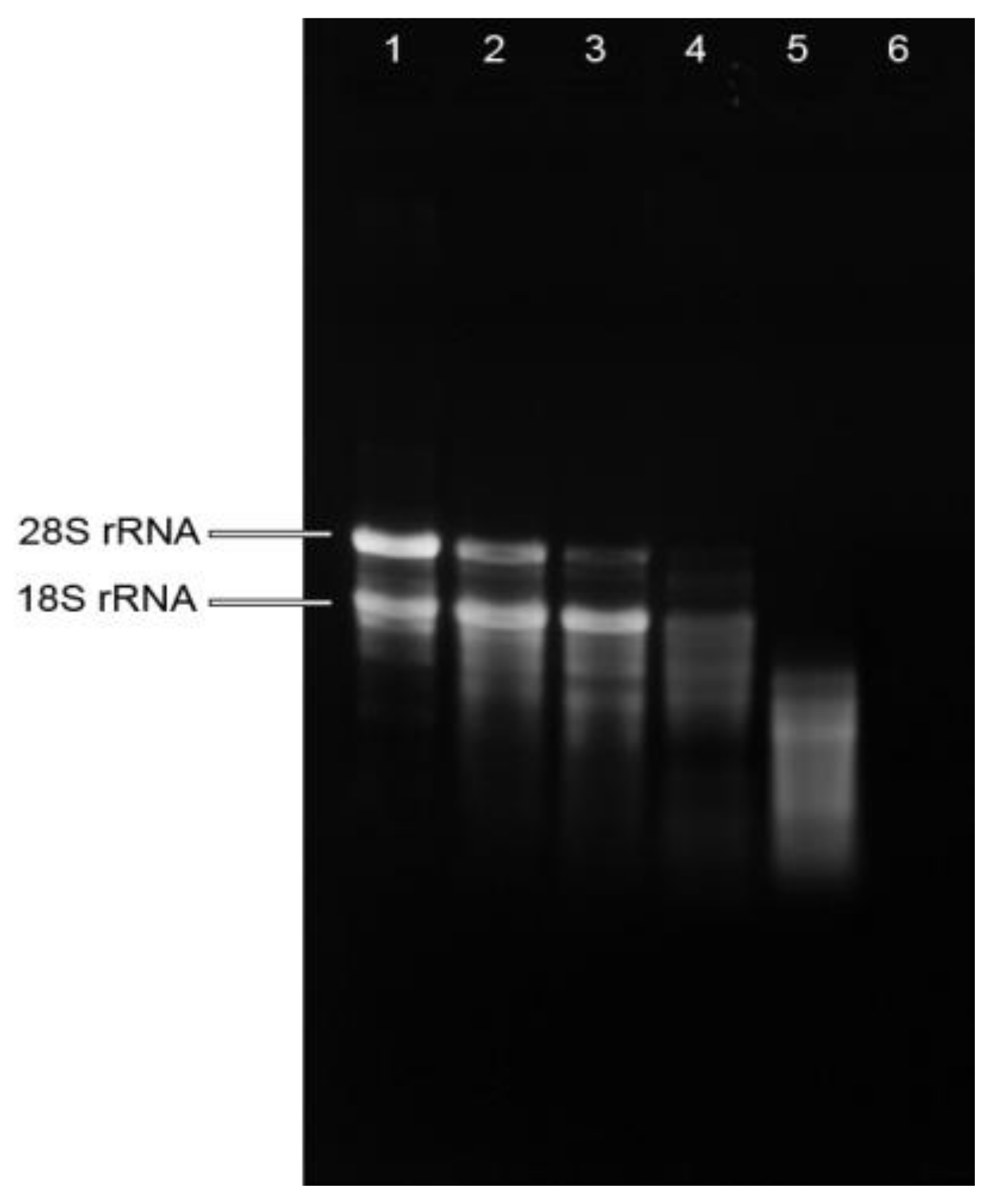

| RNA Parameters | Fresh Plant (FP) | Extract (FP), RT | Extract (FP)-Mechanical Treatment, RT | Dried Plant (DP) | Extract (DP)-Soaked, 80 °C | Extract (DP)-Boiled, 100 °C |
|---|---|---|---|---|---|---|
| RNA yield (µg/g) | 630.3 ± 73.5 | 1404.5 ± 531.2 | 1236.9 ± 482.8 | 398.2 ± 109.3 | 931.8 ± 212.7 | 26.9 ± 7.3 |
| A260/A280 | 2.10 ± 0.03 | 2.04 ± 0.05 | 2.00 ± 0.07 | 2.02 ± 0.04 | 1.97 ± 0.03 | 1.67 ± 0.11 |
| A260/A230 | 2.31 ± 0.16 | 2.44 ± 0.09 | 2.54 ± 0.09 | 2.30 ± 0.33 | 2.18 ± 0.15 | 0.80 ± 0.25 |
| Plant miRNAs | Linear Range of Ct Values | Dynamic Quantification Range of miRNA Levels | Copy Number of miRNAs per Gram of Mistletoe Plants and Lyophilized Extracts | |||||
|---|---|---|---|---|---|---|---|---|
| Fresh Plant (FP) | Extract (FP), RT | Extract (FP)-Mechanical Treatment, RT | Dried Plant (DP) | Extract (DP)-Soaked, 80 °C | Extract (DP)-Boiled, 100 °C | |||
| miR166a-3p | 13.1–28.5 | 1 nM–10 fM | 5.0 × 1011 ± 8.3 × 1010 | 7.4 × 109 ± 4.6×109 | 1.4 × 109 ± 3.0 × 108 | 1.2 × 1010 ± 6.1 × 109 | 4.2 × 108 ± 2.4 × 108 | NC |
| miR159a | 17.8–32.9 | 1 nM–10 fM | 3.9 × 1011 ± 1.2 × 1011 | 1.1 × 1010 ± 5.3 × 109 | 3.4 × 109 ± 2.0 × 109 | 1.4 × 1010 ± 3.3 × 109 | 6.3 × 107 ± 3.0 × 107 | NC |
| val-miR218 | 15.4–33.0 | 1 nM–10 fM | 7.9 × 109 ± 3.2 × 109 | 6.3 × 109 ± 3.3 × 109 | 2.0 × 109 ± 1.5 × 109 | 3.6 × 109 ± 1.8 × 109 | NC | NC |
| val-miR11 | 20.7–32.6 | 100 pM–10 fM | 1.2 × 108 ± 6.4 × 107 | 4.2 × 107 ± 2.9 × 107 | NC | 2.4 × 107 ± 1.2 × 107 | NC | NC |
| miR831-5p | 15.6–32.7 | 1 nM–1 fM | 3.3 × 107 ± 1.1 × 107 | 7.9 × 106 ± 3.3 × 106 | 2.9 × 106 ± 7.2 × 105 | 1.9 × 107 ± 6.1 × 106 | NC | NC |
| has-let-7a | 20.9–32.7 | 100 pM–10 fM | NC | NC | NC | NC | NC | NC |
| Synthetic miRNAs | Control | Mistletoe Extracts Containing Synthetic miRNAs | |||
|---|---|---|---|---|---|
| Extract (FP), RT | Extract (FP)-Mechanical Treatment, RT | Extract (DP)-Soaked, 80 °C | Extract (DP)-Boiled, 100 °C | ||
| hsa-let-7a | 23.34 ± 0.47 | 34.81 ± 0.32 | 35.78 ± 0.79 | 35.63 ± 0.35 | 37.71 ± 0.58 |
| 2′-OMe hsa-let-7a | 23.08 ± 0.43 | 33.09 ± 0.24 | 35.30 ± 0.30 | 35.28 ± 0.70 | 37.14 ± 0.69 |
| miRNAs | Sequence | RT Primer | Primer |
|---|---|---|---|
| miR166a-3p | UCGGACCAGGCUUCAUUCCCC | GTCGTATCCAGTGCGTGTCGTGGAGTCGGC AATTGCACTGGATACGACGGGGAA | F: AGTCGGACCAGGCTTCA R: CAGTGCGTGTCGTGGAG |
| miR159a | UUUGGAUUGAAGGGAGCUCU | GTCGTATCCAGTGCGTGTCGTGGAGTCGGC AATTGCACTGGATACGACAGAGCT | F: GCCGTTTGGATTGAAGG R: CAGTGCGTGTCGTGGAG |
| miR831-5p | AGGAAGACUGUAGAAGAGAUGAGG | GTCGTATCCAGTGCGTGTCGTGGAGTCGGC AATTGCACTGGATACGACCCTCAT | F: CTCAGGAAGACTGTAGAAGA R: CAGTGCGTGTCGTGGAG |
| val-miR218 | GAUGAUCGCCACGUCGGAGGA | GTCGTATCCAGTGCAGGGTCCGAGGTATTC GCACTGGATACGACTCCTCC | F: AGGGATGATCGCCACG R: GTGCAGGGTCCGAGGT |
| val-miR11 | CACUGUAGCACUUUUGACAAAG | GTCGT ATCCA GTGCA GGGTC CGAGG TATTC GCACT GGATA CGAC CTTTGT | F: GGGCACTGTAGCACTTTTG R: GTGCAGGGTCCGAGGT |
| hsa-let-7a | UGAGGUAGUAGGUUGUAUAGUU | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACTAT | F: GCCGTGAGGTAGTAGGTTGT R: GTGCAGGGTCCGAGGT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Melzig, M.F. The Stability of Medicinal Plant microRNAs in the Herb Preparation Process. Molecules 2018, 23, 919. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23040919
Xie W, Melzig MF. The Stability of Medicinal Plant microRNAs in the Herb Preparation Process. Molecules. 2018; 23(4):919. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23040919
Chicago/Turabian StyleXie, Wenyan, and Matthias F. Melzig. 2018. "The Stability of Medicinal Plant microRNAs in the Herb Preparation Process" Molecules 23, no. 4: 919. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23040919




