The Selectivity of Polymers Imprinted with Amines
Abstract
:1. Introduction
2. Theory
2.1. Definitions of MIP Selectivity
2.2. The Selectivity Ladder: A Simple Method to Show MIP Selectivity Patterns
3. Results and Discussion
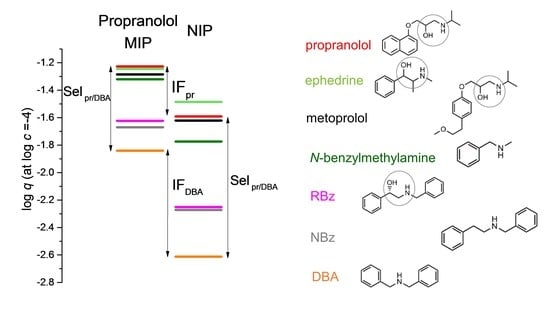
3.1. Comparison between the MIP and Its NIP
3.2. Comparison between MIPs of Different Templates
3.3. Comparison between Different Rebinding Media
4. Materials and Methods
4.1. Materials
4.2. Instrumentation
4.3. Polymer Preparation
4.4. Equilibrium Binding Experiments
5. Conclusions
- It is difficult to define, let alone measure, the selectivity of MIPs, in such a way that the obtained selectivity value could be generally used in all applications of the MIP. In other words, there is no such thing as “the” selectivity of an MIP.
- For fundamental studies of the imprinting effect, it is useful to do equilibrium binding experiments in the porogen. In other media, selectivities may be quite different.
- If the adsorption isotherms of different substances in a particular medium are approximately parallel on the log c-log q plot, it is useful to study the selectivity ladder. The selectivity ladders of different polymers, or of the same polymer in different media, may also be compared.
- When demonstrating the good selectivity of an MIP against a compound “similar” to the template, one should be aware that great similarity in the structural formulas may hide big differences in some properties (like hydrophobicity), which are relevant for adsorption.
- Imprinting, even if successful in increasing the binding of the template against the NIP, does not necessarily lead to improved selectivity compared to the NIP. Imprinting by a particular compound (the template) will often increase the binding of other compounds as well, eventually more than for the template, thus leading to reduced selectivity.
- The selectivity of MIPs in real life applications needs to be carefully studied. Demonstration of selectivity in a single experiment may be insufficient.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MIPdatabase. Available online: https://mipdatabase.com/index.php (accessed on 27 February 2017).
- Martin-Esteban, A. Molecularly-imprinted polymers as a versatile, highly selective tool in sample preparation. Trends Anal. Chem. 2013, 45, 169–181. [Google Scholar] [CrossRef]
- Beltran, A.; Borrull, F.; Cormack, P.A.G.; Marce, R.M. Molecularly-imprinted polymers: Useful sorbents for selective extractions. Trends Anal. Chem. 2010, 29, 1363–1375. [Google Scholar] [CrossRef]
- Toth, B.; Horvai, G. Chromatography, Solid-Phase Extraction, and Capillary Electrochromatography with MIPs. In Molecular Imprinting. Topics in Current Chemistry; Haupt, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 325, pp. 267–306. ISBN 978-3-642-28420-5. [Google Scholar]
- Renkecz, T.; Horvath, V.; Horvai, G. Molecularly imprinted polymers for chromatography and related techniques. In Handbook of Molecularly Imprinted Polymers, 1st ed.; Alvarez-Lorenzo, C., Concheiro, A., Eds.; Smithers Rapra: Shrewsbury, UK, 2013; pp. 141–188. ISBN 9781847359599. [Google Scholar]
- Ge, Y.; Turner, A.P.F. Molecularly Imprinted Sorbent Assays: Recent Developments and Applications. Chem. Eur. J. 2009, 15, 8100–8107. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Pietrzyk-Le, A.; D’Souza, F.; Kutner, W. Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem. 2012, 402, 3177–3204. [Google Scholar] [CrossRef] [PubMed]
- Suriyanarayanan, S.; Cywinski, P.J.; Moro, A.J.; Mohr, G.J.; Kutner, W. Chemosensors based on molecularly imprinted polymers. In Molecular Imprinting. Topics in Current Chemistry; Haupt, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 325, pp. 165–265. ISBN 978-3-642-28420-5. [Google Scholar]
- Moreno-Bondi, M.C.; Navarro-Villoslada, F.; Benito-Pena, E.; Urraca, J.L. Molecularly Imprinted Polymers as Selective Recognition Elements in Optical Sensing. Curr. Anal. Chem. 2008, 4, 316–340. [Google Scholar] [CrossRef]
- Moreno-Bondi, M.C.; Benito-Pena, M.E.; Urraca, J.L.; Orellana, G. Immuno-Like Assays and Biomimetic Microchips. In Molecular Imprinting. Topics in Current Chemistry; Haupt, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 325, pp. 111–164. ISBN 978-3-642-28420-5. [Google Scholar]
- Dorko, Z.; Tamas, B.; Horvai, G. Isotherm charts for material selection and method development with molecularly imprinted polymers and other sorbents. Talanta 2017, 162, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Schott, B.; Riedel, D.; Mizaikoff, B. Computational and experimental study on the influence of the porogen on the selectivity of 4-nitrophenol molecularly imprinted polymers. Anal. Chim. Acta 2012, 744, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Baggiani, C.; Anfossi, L.; Baravalle, P.; Giovannoli, C.; Tozzi, C. Selectivity features of molecularly imprinted polymers recognising the carbamate group. Anal. Chim. Acta 2005, 531, 199–207. [Google Scholar] [CrossRef]
- Yu, C.; Mosbach, K. Influence of mobile phase composition and cross-linking density on the enantiomeric recognition properties of molecularly imprinted polymers. J. Chromatogr. A 2000, 888, 63–72. [Google Scholar] [CrossRef]
- Spivak, D.A.; Shea, K.J. Investigation into the scope and limitations of molecular imprinting with DNA molecules. Anal. Chim. Acta 2001, 435, 65–74. [Google Scholar] [CrossRef]
- Kim, H.J.; Guiochon, G. Thermodynamic studies of the solvent effects in chromatography on molecularly imprinted polymers. 3. Nature of the organic mobile phase. Anal. Chem. 2005, 77, 2496–2504. [Google Scholar] [CrossRef] [PubMed]
- Spivak, D.; Gilmore, M.A.; Shea, K.J. Evaluation of binding and origins of specificity of 9-ethyladenine imprinted polymers. J. Am. Chem. Soc. 1997, 119, 4388–4393. [Google Scholar] [CrossRef]
- Shimizu, K.D.; Stephenson, C.J. Molecularly imprinted polymer sensor arrays. Curr. Opin. Chem. Biol. 2010, 14, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Billing, J.; Nilsson, C.; Boyd, B.; Kecili, R.; Nivhede, D.; Axelsson, S.; Rees, A. Utilizing the Cross-Reactivity of MIPs. In Molecularly Imprinted Polymers in Biotechnology. Advances in Biochemical Engineering/Biotechnology; Mattiasson, B., Ye, L., Eds.; Springer: Cham, Switzerland, 2015; Volume 150, pp. 167–182. ISBN 978-3-319-20728-5. [Google Scholar]
- Greene, N.T.; Shimizu, K.D. Colorimetric molecularly imprinted polymer sensor array using dye displacement. J. Am. Chem. Soc. 2005, 127, 5695–5700. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Pap, T.; Horvath, V.; Horvai, G. Nonlinear adsorption isotherm as a tool for understanding and characterizing molecularly imprinted polymers. J. Chromatogr. A 2006, 1119, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Pap, T.; Horvath, V.; Horvai, G. Which molecularly imprinted polymer is better? Anal. Chim. Acta 2007, 591, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Den Boef, G.; Hulanicki, A. Recommendations for the Usage of Selective, Selectivity and Related Terms in Analytical-Chemistry. Pure Appl. Chem. 1983, 55, 553–556. [Google Scholar] [CrossRef]
- Vessman, J.; Stefan, R.I.; Van Staden, J.F.; Danzer, K.; Lindner, W.; Burns, D.T.; Fajgelj, A.; Muller, H. Selectivity in analytical chemistry—(IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1381–1386. [Google Scholar] [CrossRef]
- Lanza, F.; Sellergren, B. Method for synthesis and screening of large groups of molecularly imprinted polymers. Anal. Chem. 1999, 71, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Buhlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef] [PubMed]
- Dorko, Z.; Szakolczai, A.; Toth, B.; Horvai, G. Relationship between commonly used adsorption isotherm equations impedes isotherm selection. Period. Polytech. Chem. Eng. 2017, 61, 10–14. [Google Scholar] [CrossRef]
- Andersson, L.I. Application of molecular imprinting to the development of aqueous buffer and organic solvent based radioligand binding assays for (S)-propranolol. Anal. Chem. 1996, 68, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Drug Bank. Available online: https://www.drugbank.ca/drugs (accessed on 3 May 2018).
- Baggiani, C.; Giovannoli, C.; Anfossi, L.; Passini, C.; Baravalle, P.; Giraudi, G. A connection between the binding properties of imprinted and nonimprinted polymers: A change of perspective in molecular imprinting. J. Am. Chem. Soc. 2012, 134, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Manesiotis, P.; Hall, A.J.; Courtois, J.; Irgum, K.; Sellergren, B. An artificial riboflavin receptor prepared by a template analogue imprinting strategy. Angew. Chem. Int. Ed. 2005, 44, 3902–3906. [Google Scholar] [CrossRef] [PubMed]
- Manesiotis, P.; Hall, A.J.; Sellergren, B. Improved imide receptors by imprinting using pyrimidine-based fluorescent reporter monomers. J. Org. Chem. 2005, 70, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Dorko, Z.; Tamas, B.; Horvai, G. Relationship between Individual and Competitive Adsorption Isotherms on Molecularly Imprinted Polymers. Period. Polytech. Chem. Eng. 2017, 61, 33–38. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorkó, Z.; Nagy-Szakolczai, A.; Tóth, B.; Horvai, G. The Selectivity of Polymers Imprinted with Amines. Molecules 2018, 23, 1298. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23061298
Dorkó Z, Nagy-Szakolczai A, Tóth B, Horvai G. The Selectivity of Polymers Imprinted with Amines. Molecules. 2018; 23(6):1298. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23061298
Chicago/Turabian StyleDorkó, Zsanett, Anett Nagy-Szakolczai, Blanka Tóth, and George Horvai. 2018. "The Selectivity of Polymers Imprinted with Amines" Molecules 23, no. 6: 1298. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23061298