Indium-Mediated Allylation of Carbonyl Compounds in Ionic Liquids: Effect of Salts in Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Allylation
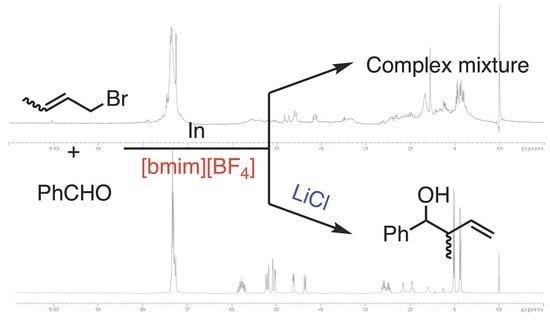
2.2. Crotylation
2.3. Intramolecular Auxiliary
2.4. Chiral Auxiliary
3. Materials and Methods
3.1. Analysis
3.2. General Procedure for the Allylation of Benzaldehyde in Ionic Liquids
3.3. General Procedure for the Pd-Catalyzed Allylation of Benzaldehyde in Ionic Liquids
3.4. General Procedure for the Crotylation of Benzaldehyde in Ionic Liquids
3.5. Crotylation of Benzaldehyde in the Presence of Additives
3.6. General Procedure for the Asymmetric Indium-Mediated Allylation of Benzaldehyde Using Cinchonidine
3.7. Product Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Araki, S.; Hirashita, T. Comprehensive Organometallic Chemistry III; Crabtree, R., Mingos, M., Eds.; Elsevier: Oxford, UK, 2007; pp. 649–751. ISBN 978-0-08-045047-6. [Google Scholar]
- Yadav, J.S.; Antony, A.; George, J.; Subba Reddy, B.V. Recent Developments in Indium Metal and Its Salts in Organic Synthesis. Eur. J. Org. Chem. 2010, 591–605. [Google Scholar] [CrossRef]
- Roy, U.K.; Roy, S. Making and Breaking of Sn-C and In-C Bonds in Situ: The Cases of Allyltins and Allylindiums. Chem. Rev. 2010, 110, 2472–2535. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, H.S.; Kim, K.H.; Kim, S.H.; Kim, J.N. Recent advances in allylindium reagents in organic synthesis. Tetrahedron 2010, 66, 7065–7076. [Google Scholar] [CrossRef]
- Araki, S.; Jin, S.-J.; Idou, Y.; Butsugan, Y. Allylation of Carbonyl Compounds with Catalytic Amount of Indium. Bull. Chem. Soc. Jpn. 1992, 65, 1736–1738. [Google Scholar] [CrossRef]
- Hirashita, T.; Sato, Y.; Yamada, D.; Takahashi, F.; Araki, S. Ionic Liquid-accelerated Allylation of Carbonyl Compounds with a Catalytic Amount of Indium Generated From in Situ Reduction of InCl3 with Aluminium. Chem. Lett. 2011, 40, 506–507. [Google Scholar] [CrossRef]
- Law, M.C.; Wong, K.-Y.; Chan, T.H. Grignard Reagents in Ionic Liquids. Chem. Commun. 2006, 2457–2459. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Atefi, F.; Singer, R.D.; Scammells, P.J. Grignard Reactions in Pyridinium and Phosphonium Ionic Liquids. Eur. J. Org. Chem. 2011, 2011, 942–950. [Google Scholar] [CrossRef]
- Gordon, C.M.; Ritchie, C. Indium and Tin-Mediated Allylation in Ionic Liquids. Green Chem. 2002, 4, 124–128. [Google Scholar] [CrossRef]
- Law, M.C.; Wong, K.-Y.; Chan, T.H. Metal Mediated Allylation of Carbonyl Compounds in Ionic Liquids. Green Chem. 2002, 4, 161–164. [Google Scholar] [CrossRef]
- Bowyer, M.C.; Gordon, C.M.; Leitch, S.K.; McCluskey, A.; Ritchie, C. Indium-mediated Addition of 4-Bromocrotonic Acid to Aldehydes and Ketones—A Simple, High Yielding Route to α-Allyl-β-hydroxy Carboxylic Acids. Aust. J. Chem. 2004, 57, 135–137. [Google Scholar] [CrossRef]
- Dey, P.; Koli, M.; Goswami, D.; Sharma, A.; Chattopadhyay, S. [bmim][Br] as an Inexpensive and Efficient Medium for the Barbier-Type Allylation Reaction Using a Catalytic Amount of Indium: Mechanistic Studies. Eur. J. Org. Chem. 2018, 2018, 1333–1341. [Google Scholar] [CrossRef]
- Law, M.C.; Cheung, T.W.; Wong, K.Y.; Chan, T.H. Synthetic and Mechanistic Studies of Indium-Mediated Allylation of Imines in Ionic Liquids. J. Org. Chem. 2007, 72, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Kamei, T.; Hirashita, T.; Yamamura, H.; Kawai, M. A New Preparative Method for Allylic Indium(III) Reagents by Reductive Transmetalation of π-Allylpalladium(II) with Indium(I) Salts. Org. Lett. 2000, 2, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Soengas, R.G.; Silva, V.L.M.; Pinto, J.; Rodríguez-Solla, H.; Silva, A.M.S. Ohmic Heating and Ionic Liquids in Combination for the Indium-Promoted Synthesis of 1-Halo Alkenyl Compounds: Applications to Pd-Catalysed Cross-Coupling Reactions. Eur. J. Org. Chem. 2016, 2016, 99–107. [Google Scholar] [CrossRef]
- The ee values of the syn and anti diastereomers were both 5%.
- One of the reviewers suggested a possibility that both crotylindium species and carbonyl compounds are activated by additives.
- Tan, K.-T.; Chng, S.-S.; Cheng, H.-S.; Loh, T.-P. Development of a Highly α-Regioselective Metal-mediated Allylation Reaction in Aqueous Media: New Mechanistic Proposal for the Origin of α-Homoallylic Alcohols. J. Am. Chem. Soc. 2003, 125, 2958–2963. [Google Scholar] [CrossRef] [PubMed]
- Paquette, L.A.; Rothhaar, R.R. Competitive Intramolecular/Intermolecular Chelation Options Operative during Indium-Promoted Additions to Pyridyl Aldehydes and to Glyoxylic Acid under Aqueous Conditions. J. Org. Chem. 1999, 64, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hirashita, T.; Kamei, T.; Satake, M.; Horie, T.; Shimizu, H.; Araki, S. Control of Diastereoselectivity in the Crotylation and Cinnamylation of Aldehydes by the Selection of Ligands on Allylic Indium Reagents. Org. Biomol. Chem. 2003, 1, 3799–3803. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.-P.; Zhou, J.-R.; Yin, Z. A Highly Enantioselective Indium-Mediated Allylation Reaction of Aldehydes. Org. Lett. 1999, 11, 1855–1857. [Google Scholar] [CrossRef]
- Kim, I.S.; Ngai, M.-Y.; Krische, M.J. Enantioselective Iridium-catalyzed Carbonyl Allylation from the Aalcohol or Aldehyde Oxidation Level Using Allyl Acetate as an Allyl Metal Surrogate. J. Am. Chem. Soc. 2008, 130, 6340–6341. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Han, S.B.; Krischa, M.J. anti-Diastereo- and Enantioselective Carbonyl Crotylation from the Alcohol or Aldehyde Oxidation Level Employing a Cyclometallated Iridium Catalyst: α-Methyl Allyl Acetate as a Surrogate to Preformed Crotylmetal Reagents. J. Am. Chem. Soc. 2009, 131, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Adele, A.; Wu, X.; Gong, L. A Highly Enantioselective Mannich-Type Reaction of Glycine Schiff Base Catalyzed by a Cinchoninium Salt. Chin. J. Chem. 2014, 32, 969–973. [Google Scholar] [CrossRef]
- Reilly, M.K.; Rychnovsky, S.D. Allyl Transfer to Aldehydes and Ketones by Brønsted Acid Activation of Allyl and Crotyl 1,3,2-Dioxazaborolidines. Org. Lett. 2010, 12, 4892–4895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, K.; Sakai, H.; Nakai, T. [2,3]-Wittig Rearrangement of Crotyl Picolyl Ethers. Evaluation of the Electronic Effect as a Stereo-Directing Factor. Chem. Lett. 1993, 8, 1397–1400. [Google Scholar] [CrossRef]
Sample Availability: Not available. |




| Entry | Ionic Liquid | Yield of 1 (%) b |
|---|---|---|
| 1 | [bmim][BF4] | 95 |
| 2 | [bpy][BF4] | 82 |
| 3 | [bmim][(CN)2N] | 88 |
| 4 | [bmim][OTf] | 69 |
| 5 c | [bmim][Cl] | 41 |
| 6 | [bmim][NTf2] | 0 |

| Entry | Ionic Liquid | Yield of 1 (%) b |
|---|---|---|
| 1 | [bmim][BF4] | 75 |
| 2 | [bpy][BF4] | 61 |
| 3 | [bmim][OTf] | 50 |
| 4 | [bmim][(CN)2N] | 16 |

| Entry | Ionic Liquid | Time (h) | Yield of 2 (%) b |
|---|---|---|---|
| 1 | [bmim][(CN)2N] | 0.5 | 80 (58:42) c |
| 2 | [bmim][OTf] | 1 | 67 (55:45) c |
| 3 | [bmim][BF4] | 1 | Complex mixture |

| Entry | Additive (mol %) | Time (h) | NMR Yield (%) (syn:anti) b |
|---|---|---|---|
| 1 | [bmim][Cl] (114) | 1 | 2: 90 (54:46) |
| 2 | LiCl (100) | 1 | 2: 92 (59:41) |
| 3 c | LiCl (10) | 1 | 2: 8 (66:34) d |
| 4 | LiCl (200) | 1 | 2: 58 (57:43) |
| 5 | [bmim][Cl] (10) | 5 | 2: 65 (58:42) |
| 6 | [bmim][Br] (10) | 5 | 2: 80 (63:37) |
| 7 | LiBr (10) | 1 | 2: 62 (63:37) |
| 8 | PPh3 (100) | 2 | 2: 78 (56:44) |
| 9 | 1-Butylamine (100) | 3 | 2: 48 (58:42) |
| 10 | TMEDA e (100) | 18 | trace |
| 11 | Pyridine (100) | 6 | 2: 75 (57:43) |
| 12 | Cinchonidine (100) | 6 | 2: 82 (54:46) |
| 13 | 1-Hexanol (100) | 6 | 2: 26 (73:27) d, 3: 41 (47:53) d,f |
| 14 | Phenol (100) | 6 | 0 |
| 15 | Diethyl l-tartrate (100) | 3 | 2: 19 (75:25) d, 3: 33 (55:45) d,f |
| 16 | H2O (100) | 6 | 2: 45 (74:26) d, 3: 42 (48:52) d,f |

| Entry | Solvent Solvent 1/Solvent 2 | Temp. (°C) | Time (h) | Yield (%) | Ee (%) b |
|---|---|---|---|---|---|
| 1 | [bmim][BF4] | rt | 15 | 50 | 2 |
| 2 | [bmim][BF4]/CH2Cl2 | rt | 15 | 90 | 33 |
| 3 | [bmim][BF4]/CH2Cl2 | −78 | 15 | 41 | 2 |
| 4 | [bmim][BF4]/CH2Cl2 | 40 | 15 | 51 | 20 |
| 5 c | [bmim][BF4]/CH2Cl2 | rt | 1 | 81 | 6 |
| 6 | [bpy][BF4]/CH2Cl2 | rt | 15 | 71 | 28 |
| 7 | [bpy][BF4]/CH2Cl2 | −60 | 65 | 76 | 3 |
| 8 | [bmim][OTf] | rt | 15 | 81 | 0 |
| 9 | [bmim][OTf]/CH2Cl2 | rt | 1 | 98 | 51 |
| 10 | [bmim][OTf]/CH2Cl2 | −20 | 15 | 23 | 62 |
| 11 | [bmim][OTf]/CH2Cl2 | −78 | 15 | 34 | 61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirashita, T.; Takahashi, F.; Noda, T.; Takagi, Y.; Araki, S. Indium-Mediated Allylation of Carbonyl Compounds in Ionic Liquids: Effect of Salts in Ionic Liquids. Molecules 2018, 23, 1696. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23071696
Hirashita T, Takahashi F, Noda T, Takagi Y, Araki S. Indium-Mediated Allylation of Carbonyl Compounds in Ionic Liquids: Effect of Salts in Ionic Liquids. Molecules. 2018; 23(7):1696. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23071696
Chicago/Turabian StyleHirashita, Tsunehisa, Fusako Takahashi, Takayuki Noda, Yuji Takagi, and Shuki Araki. 2018. "Indium-Mediated Allylation of Carbonyl Compounds in Ionic Liquids: Effect of Salts in Ionic Liquids" Molecules 23, no. 7: 1696. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23071696