Pumilacidins from the Octocoral-Associated Bacillus sp. DT001 Display Anti-Proliferative Effects in Plasmodium falciparum
Abstract
:1. Introduction
2. Results
2.1. Collection and Identification of Biological Material
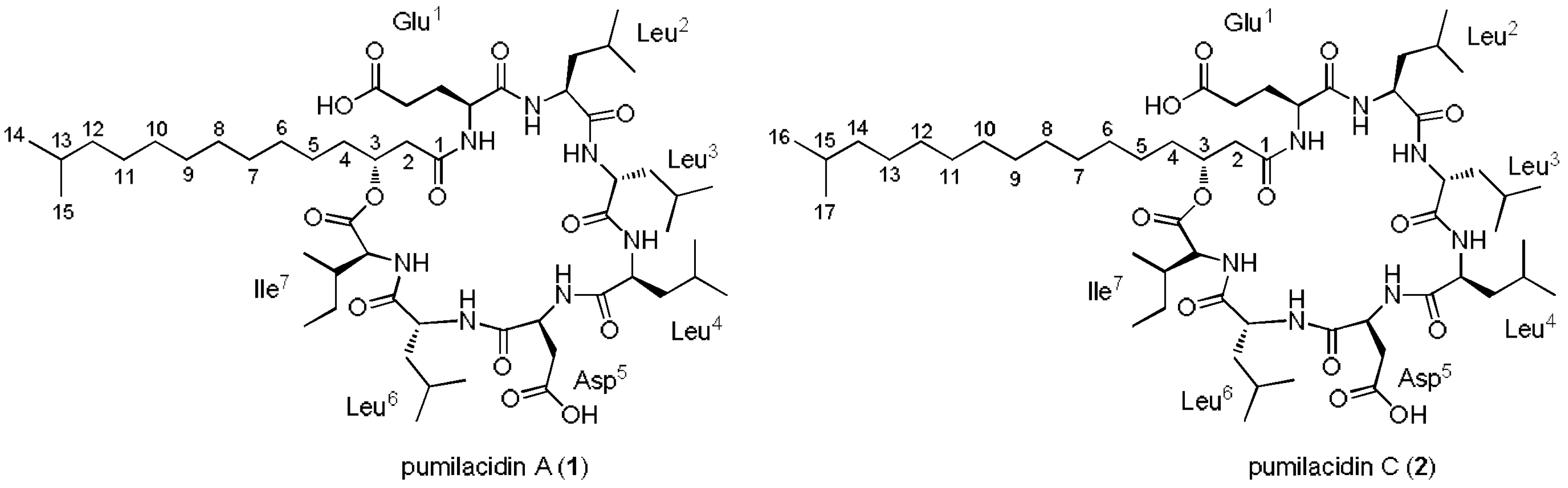
2.2. Extraction, Isolation, and Characterization of Compounds
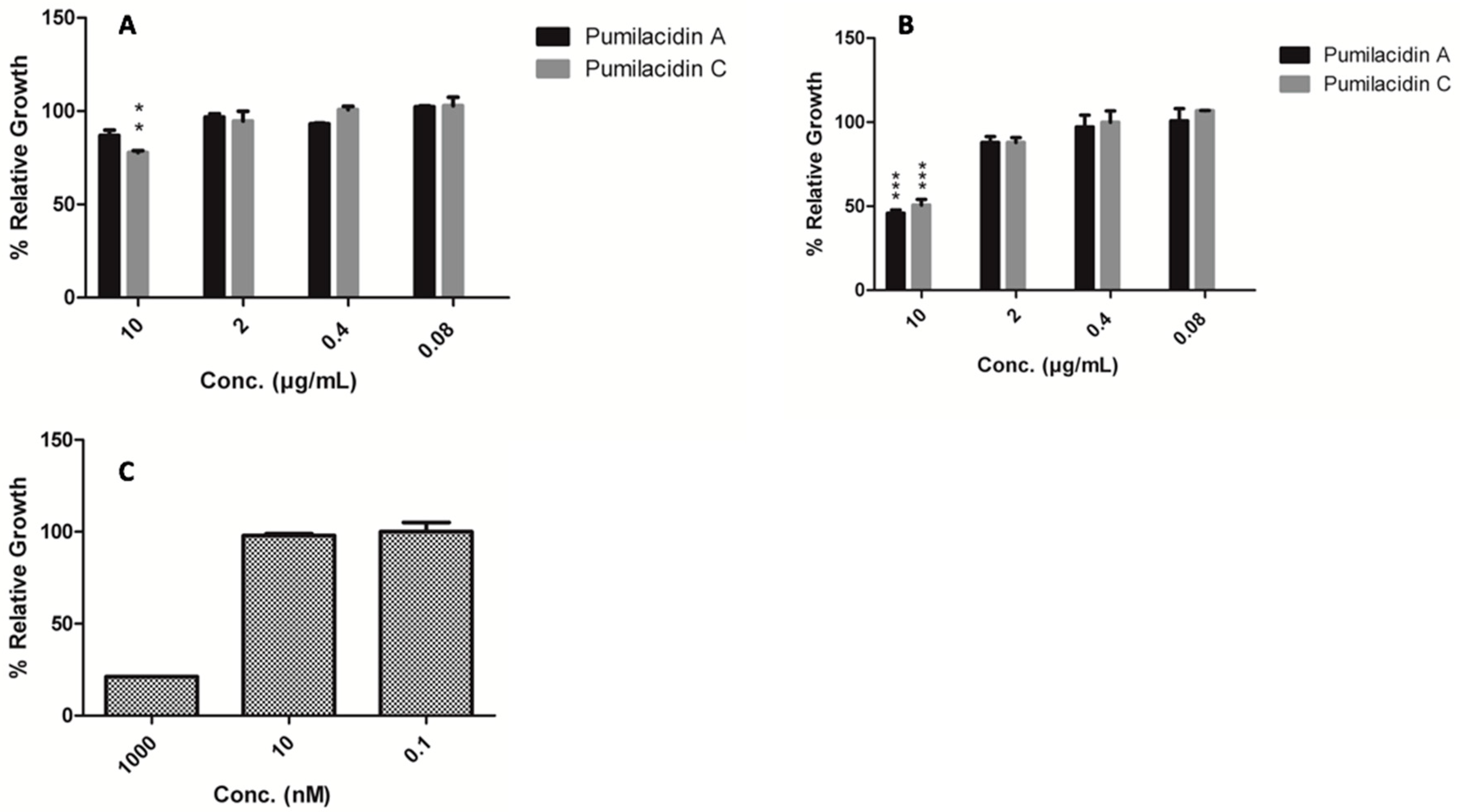
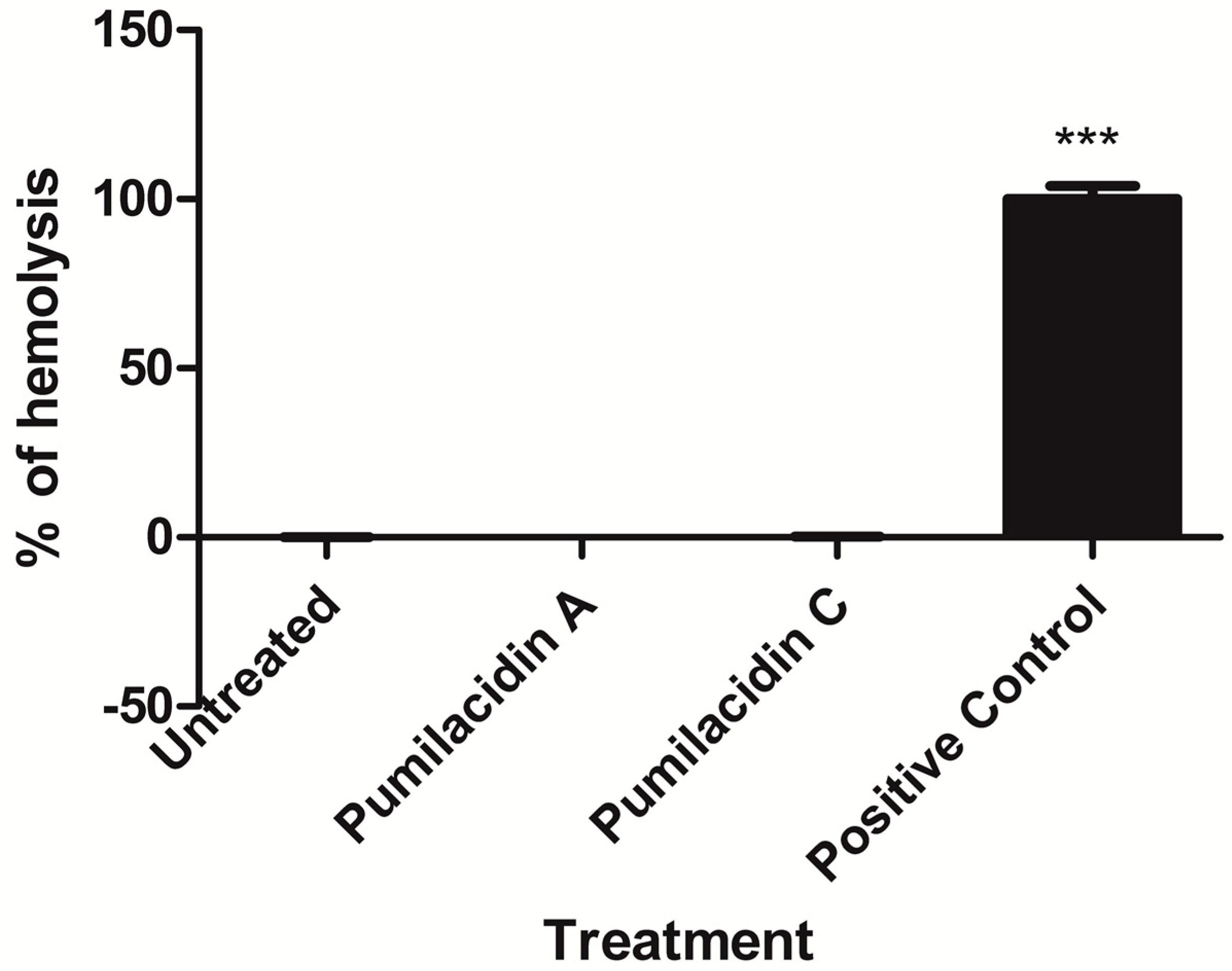
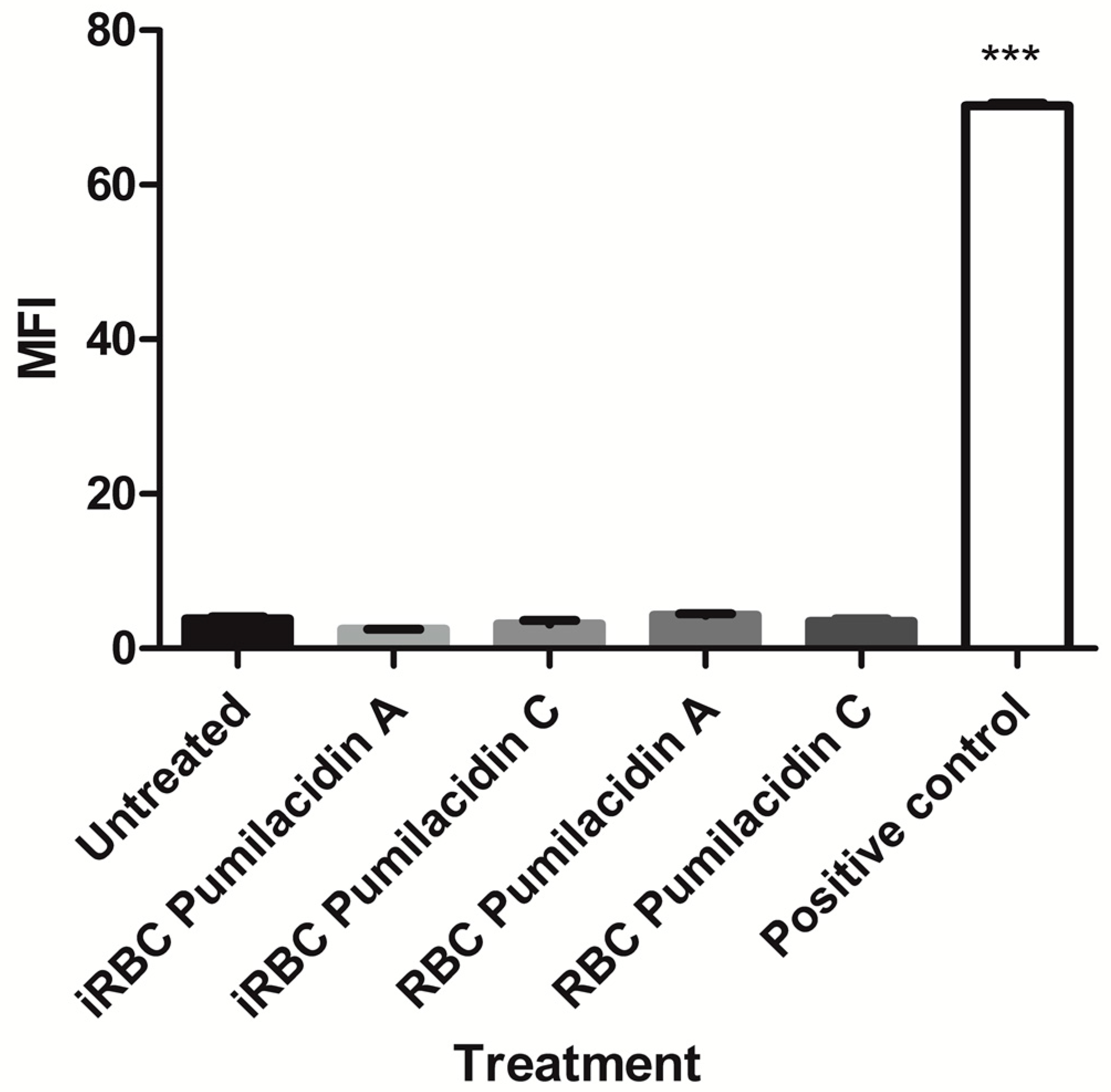
2.3. Pumilacidins Inhibit the Growth of Plasmodium falciparum Parasites without Affecting Uninfected Cells
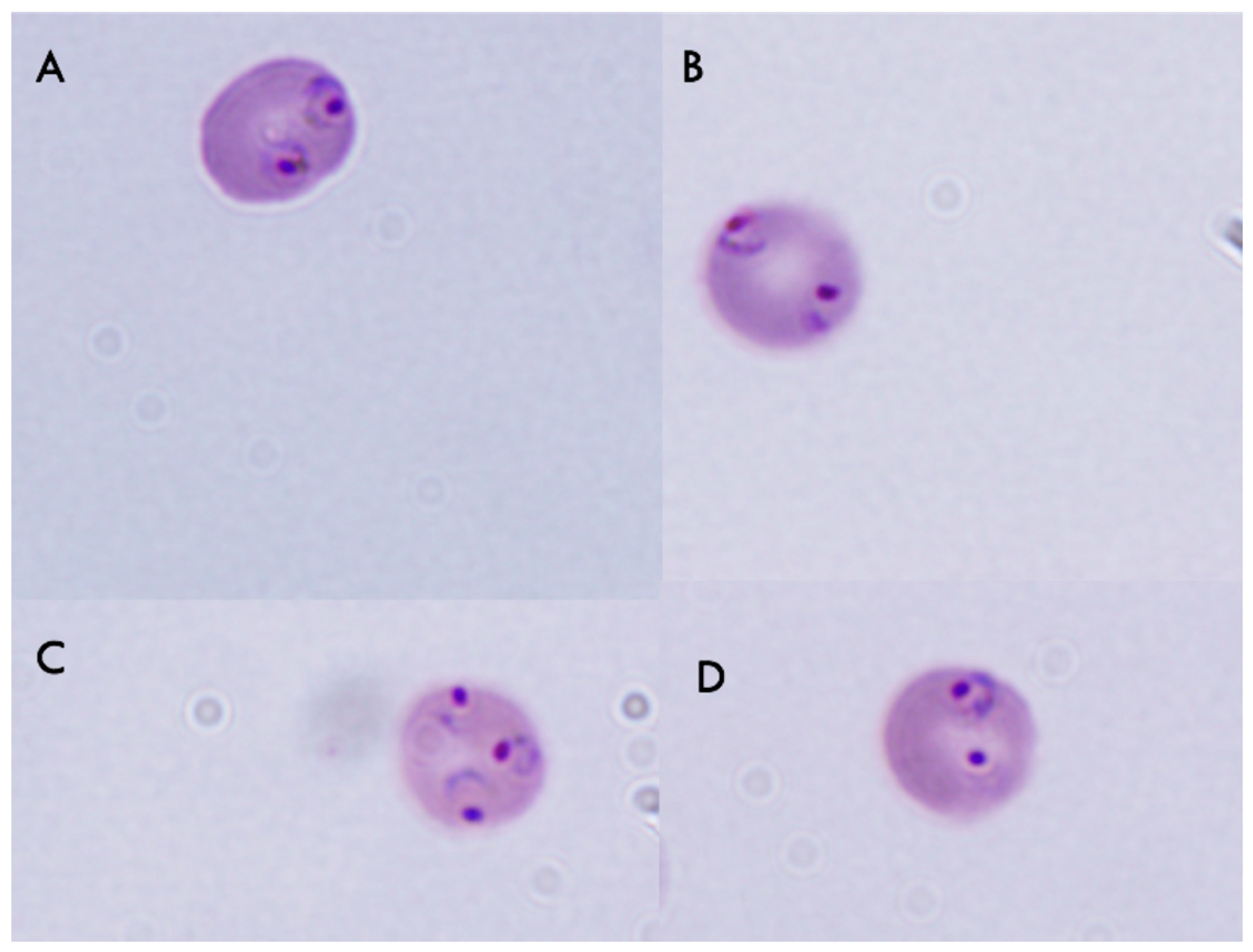
2.4. Effect of Pumilacidins on the Induction of Pro-Apoptotic-Like Activity
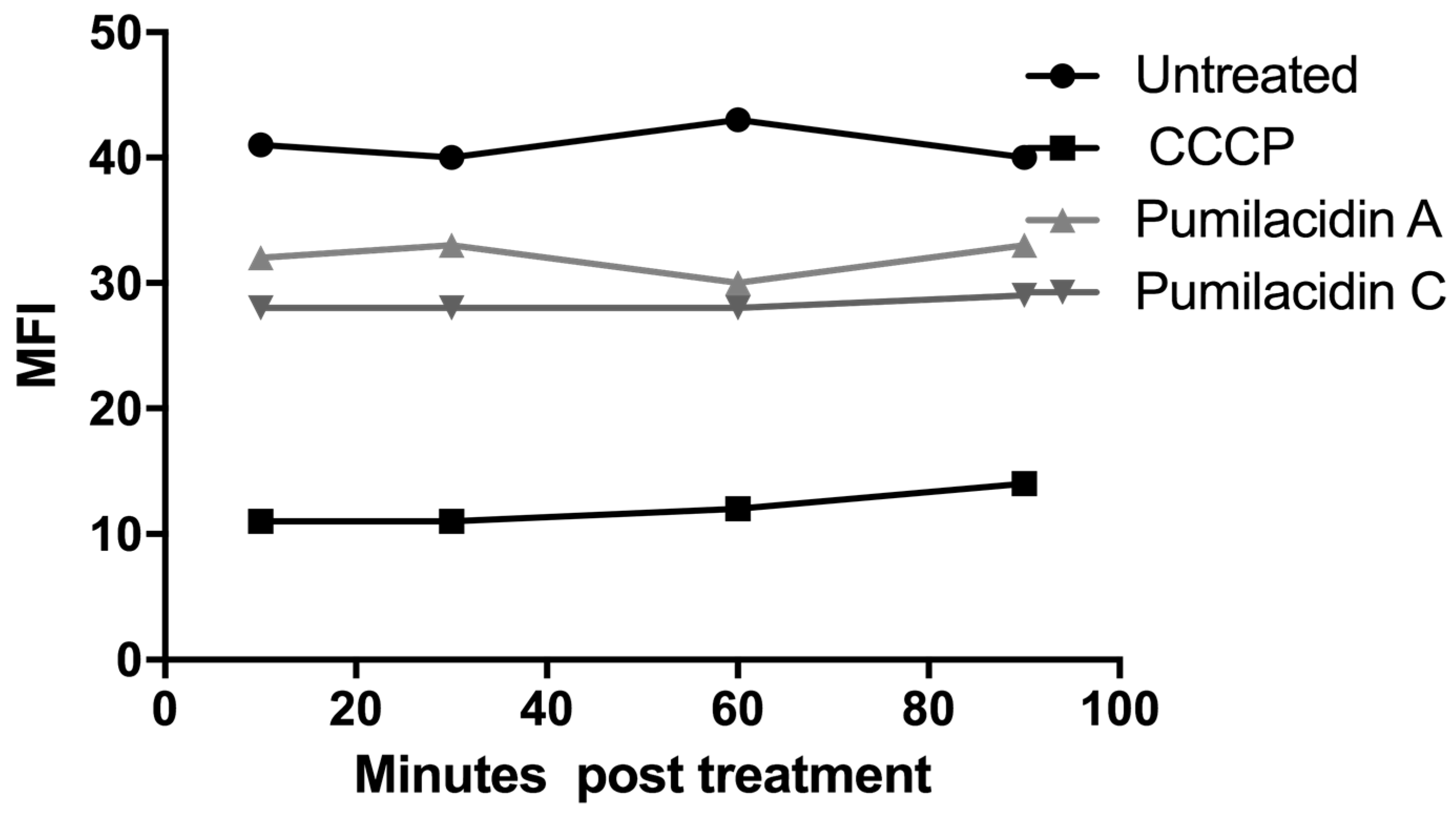
2.5. Pumilacidin-Induced Apoptosis-Like Death through Mitochondrial Dysfunction Pathways
2.6. Reactive Oxygen Species Generation Has No Role in the Effect of Pumilacidins on Parasites
2.7. Calcium Involvement in Parasite Death after Pumilacidin Treatment
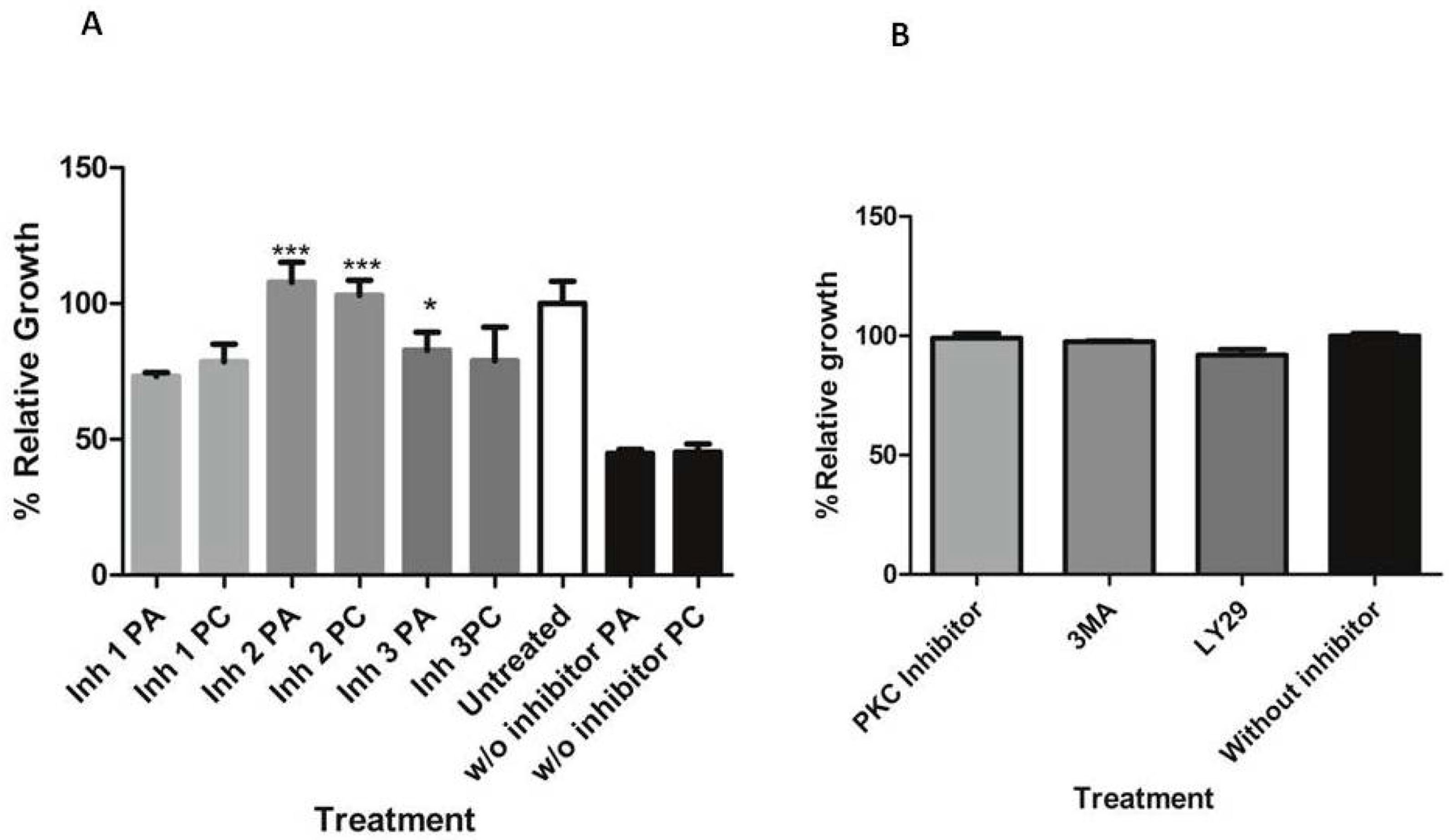
2.8. Effect of Pumilacidins on the Activation of Inositol Trisphosphate Apoptosis-Regulatory Signals
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Biological Material Collection and Identification
4.3. Fermentation, Extraction, and Isolation of Surfactins
4.4. Tandem Mass Spectrometry Experiments
4.5. Parasites and Cultures
4.6. Cell Viability Assays
4.7. IC50 Calculation for Pumilacidins A and C
4.8. Anti Plasmodium Assays
4.9. Hemolysis Assay
4.10. Propidium Iodide (PI) Incorporation Assay
4.11. Mitochondrial Membrane Potential (∆ψ) Measurements in P. falciparum
4.12. Intracellular Reactive Oxygen Species (ROS) Production in P. falciparum
4.13. Calcium Measurements
4.14. Transduction Pathways
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Cameotra, S.S. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 2004, 22, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; de Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef] [PubMed]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and Mechanistic Insights into the Direct and Indirect Antimicrobial Properties of Bacillus subtilis Lipopeptides on Plant Pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.A.M.; Shin, H.J.; Islam, M.T. Diversity of secondary metabolites from marine Bacillus species: Chemistry and Biological activity. Mar. Drugs 2013, 11, 2846–2872. [Google Scholar] [CrossRef] [PubMed]
- Hee, G.; Kim, J.; Muthaiya, M.J.; Lee, S.; Kim, H.Y.; Lee, C.H. Antimicrobial Compounds Profile During Cheonggukjang Fermentation Against Xanthomonas oryzae pv. oryzae (Xoo). J. Microbiol. Biotechnol. 2011, 21, 1147–1150. [Google Scholar]
- Brack, C.; Mikolasch, A.; Schlueter, R.; Otto, A.; Becher, D.; Wegner, U.; Albrecht, D.; Riedel, K.; Schauer, F. Antibacterial Metabolites and Bacteriolytic Enzymes Produced by Bacillus pumilus During Bacteriolysis of Arthrobacter citreus. Mar. Biotechnol. 2015, 17, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Vitullo, D.; Senatore, M.; Lima, G.; Lanzotti, V. Antifungal cyclic lipopeptides from Bacillus amyloliquefaciens strain BO5A. J. Nat. Prod. 2013, 76, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Vitullo, D.; Senatore, M.; Lima, G.; Lanzotti, V. Antifungal cyclic lipopeptides from Bacillus amyloliquefaciens strain BO7. J. Nat. Prod. 2011, 74, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.V.; Bose, A.; Keharia, H. Identification and characterization of novel surfactins produced by fungal antagonist Bacillus amyloliquefaciens 6B. Biotechnol. Appl. Biochem. 2014, 61, 349–356. [Google Scholar] [PubMed]
- Naruse, N.; Tenmyo, O.; Kobaru, S.; Kamei, H.; Miyaki, T.; Konishi, M.; Oki, T. Pumilacidin, a complex of new antiviral antibiotics. Production, isolation, chemical properties, structure and biological activity. J. Antibiot. 1990, 43, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.; Deleu, M.; Nott, K.; Wathelet, B.; Thonart, P.; Paquot, M. Hemolytic activity of new linear surfactin analogs in relation to their physico-chemical properties. Biochim. Biophys. Acta Gen. Subj. 2005, 1726, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Ng, T.B.; Yuan, F.; Liu, Z.K.; Liu, F. Induction of apoptosis in human leukemia K562 cells by cyclic lipopeptide from Bacillus subtilis natto T-2. Peptides 2007, 28, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Vollenbroich, D.; Pauli, G.; Özel, M.; Vater, J. Antimycoplasma properties and application in cell culture of surfactin, a lipopeptide antibiotic from Bacillus subtilis. Appl. Environ. Microbiol. 1997, 63, 44–49. [Google Scholar] [PubMed]
- Kim, S.D.; Cho, J.Y.; Park, H.J.; Lim, C.R.; Lim, J.H.; Yun, H.I.; Park, S.C.; Kim, S.K.; Rhee, M.H. A comparison of the anti-inflammatory activity of surfactin A, B, C, and D from Bacillus subtilis. J. Microbiol. Biotechnol. 2006, 16, 1656–1659. [Google Scholar]
- Tang, J.S.; Zhao, F.; Gao, H.; Dai, Y.; Yao, Z.H.; Hong, K.; Li, J.; Ye, W.C.; Yao, X.S. Characterization and online detection of surfactin isomers based on HPLC-MS n analyses and their inhibitory effects on the overproduction of nitric oxide and the release of TNF-α and IL-6 in LPS-induced macrophages. Mar. Drugs 2010, 8, 2605–2618. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.E.; Lee, Y.G.; Kim, B.H.; Shen, T.; Lee, S.Y.; Park, H.J.; Park, S.C.; Rhee, M.H.; Cho, J.Y. Surfactin blocks NO production in lipopolysaccharide-activated macrophages by inhibiting NF-κB activation. J. Microbiol. Biotechnol. 2008, 18, 1984–1989. [Google Scholar] [PubMed]
- Zhang, Y.; Liu, C.; Dong, B.; Ma, X.; Hou, L.; Cao, X.; Wang, C. Anti-inflammatory Activity and Mechanism of Surfactin in Lipopolysaccharide-Activated Macrophages. Inflammation 2015, 38, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Assié, L.K.; Deleu, M.; Arnaud, L.; Paquot, M.; Thonart, P.; Gaspar, C.; Haubruge, E. Insecticide activity of surfactins and iturins from a biopesticide Bacillus subtilis Cohn (S499 strain). Meded. Rijksuniv. Gent. Fak. Landbouwkd. Toegep. Biol. Wet. 2002, 67, 647–655. [Google Scholar] [PubMed]
- Park, S.Y.; Kim, J.-H.; Lee, S.J.; Kim, Y. Surfactin exhibits neuroprotective effects by inhibiting amyloid β-mediated microglial activation. Neurotoxicology 2013, 38, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the antifungal and antibacterial agents: Applications in food safety and therapeutics. BioMed Res. Int. 2015, 2015, 473050. [Google Scholar] [CrossRef] [PubMed]
- Saggese, A.; Culurciello, R.; Casillo, A.; Corsaro, M.M.; Ricca, E.; Baccigalupi, L. A Marine Isolate of Bacillus pumilus Secretes a Pumilacidin Active against Staphylococcus aureus. Mar. Drugs 2018, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Sow, F.; Nyonda, M.; Bienvenu, A.-L.; Picot, S. Wanted Plasmodium falciparum, dead or alive. Microb. Cell 2015, 2, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Meslin, B.; Barnadas, C.; Boni, V.; Latour, C.; De Monbrison, F.; Kaiser, K.; Picot, S. Features of apoptosis in Plasmodium falciparum erythrocytic stage through a putative role of PfMCA1 metacaspase-like protein. J. Infect. Dis. 2007, 195, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Deponte, M. Programmed cell death in protists. Biochim. Biophys. Acta 2008, 1783, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Ch’ng, J.-H.; Yeo, S.-P.; Shyong-Wei Tan, K. Can a single “powerless” mitochondrion in the malaria parasite contribute to parasite programmed cell death in the asexual stages? Mitochondrion 2013, 13, 254–256. [Google Scholar] [CrossRef] [PubMed]
- López, M.L.; Vommaro, R.; Zalis, M.; de Souza, W.; Blair, S.; Segura, C. Induction of cell death on Plasmodium falciparum asexual blood stages by Solanum nudum steroids. Parasitol. Int. 2010, 59, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Dragovich, T.; Rudin, C.M.; Thompson, C.B. Signal transduction pathways that regulate cell survival and cell death. Oncogene 1998, 17, 3207–3213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Asanuma, T.; Niwa, K.; Inanami, O. Regulation of Cell Survival and Death Signals Induced by Oxidative Stress. J. Clin. Biochem. Nutr. 2008, 43, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarty, S.P.; Saikumari, Y.K.; Bopanna, M.P.; Balaram, H. Biochemical characterization of Plasmodium falciparum Sir2, a NAD+-dependent deacetylase. Mol. Biochem. Parasitol. 2008, 158, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Religa, A.A.; Waters, A.P. Sirtuins of parasitic protozoa: In search of function(s). Mol. Biochem. Parasitol. 2012, 185, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Mancio-Silva, L.; Lopez-Rubio, J.J.; Claes, A.; Scherf, A. Sir2a regulates rDNA transcription and multiplication rate in the human malaria parasite Plasmodium falciparum. Nat. Commun. 2013, 4, 1530. [Google Scholar] [CrossRef] [PubMed]
- Tonkins, C.J.; Carret, C.K.; Duraisingh, M.T.; Voss, T.S.; Ralph, S.A.; Hommel, M.; Duffy, M.F.; da Silva, L.M.; Scherf, A.; Ivens, A.; et al. Sir2 Paralogues Cooperate to Regulate Virulence Genes and Antigenic Variation in Plasmodium falciparum. PLoS Biol. 2009, 7, e1000084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duraisingh, M.T.; Voss, T.S.; Marty, A.J.; Duffy, M.F.; Good, R.T.; Thompson, J.K.; Freitas-Junior, L.H.; Scherf, A.; Crabb, B.S.; Cowman, A.F. Heterochromatin Silencing and Locus Repositioning Linked to Regulation of Virulence Genes in Plasmodium falciparum. Cell 2005, 121, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, J.Y.; Kim, S.H.; Bae, H.J.; Yi, H.; Yoon, S.H.; Koo, B.S.; Kwon, M.; Cho, J.Y.; Lee, C.E.; et al. Surfactin from Bacillus subtilis displays anti-proliferative effect via apoptosis induction, cell cycle arrest and survival signaling suppression. FEBS Lett. 2007, 581, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kong, Q.; Qin, C.; Chen, Y.; Chen, Y.; Lv, R.; Zhou, G. Identification of lipopeptides in Bacillus megaterium by two-step ultrafiltration and LC-ESI-MS/MS. AMB Express 2016, 6, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-S.; Gao, H.; Hong, K.; Yu, Y.; Jiang, M.-M.; Lin, H.-P.; Ye, W.-C.; Yao, X.-S. Complete assignments of (1)H and (13)C NMR spectral data of nine surfactin isomers. Magn. Reson. Chem. 2007, 45, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, B.P.; Burke, J.E.; Masson, G.; Williams, R.L.; Falke, J.J. Regulation of PI3K by PKC and MARCKS: Single-Molecule Analysis of a Reconstituted Signaling Pathway. Biophys. J. 2016, 110, 1811–1825. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Jain, S.; Sinha, D.; Gupta, M.; Asad, M.; Srivastava, A.; Narayanan, M.S.; Ramasamy, G.; Chauhan, V.S.; Gupta, D. Mohmmed, a Disruption of a mitochondrial protease machinery in Plasmodium falciparum is an intrinsic signal for parasite cell death. Cell Death Dis. 2011, 2, e231. [Google Scholar] [CrossRef] [PubMed]
- Vaid, A.; Ranjan, R.; Smythe, W.A.; Hoppe, H.C.; Sharma, P. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum, is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood 2010, 115, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Cottingham, C.; Rayner, J.C. Effects of calcium signaling on Plasmodium falciparum erythrocyte invasion and post-translational modification of gliding-associated protein 45 (PfGAP45). Mol. Biochem. Parasitol. 2009, 168, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.M.; Adams, J.H.; Silva, J.C.; Bidwell, S.L.; Lorenzi, H.; Caler, E.; Crabtree, J.; Angiuoli, S.V.; Merino, E.F.; Amedeo, P.; et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature 2008, 455, 757–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doerig, C.D. Signal transduction in malaria parasites. Parasitol. Today 1997, 13, 307–313. [Google Scholar] [CrossRef]
- Hall, B.S.; Daramola, O.O.; Barden, G.; Targett, G.A.T. Modulation of Protein Kinase C Activity in Plasmodium falciparum—Infected Erythrocytes. Blood 1997, 89, 1770–1778. [Google Scholar] [PubMed]
- Wu, Y.T.; Tan, H.L.; Shui, G.; Bauvy, C.; Huang, Q.; Wenk, M.R.; Ong, C.N.; Codogno, P.; Shen, H.M. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J. Biol. Chem. 2010, 285, 10850–10861. [Google Scholar] [CrossRef] [PubMed]
- Mbengue, A.; Bhattacharjee, S.; Pandharkar, T.; Liu, H.; Estiu, G.; Stahelin, R.V.; Rizk, S.S.; Njimoh, D.L.; Ryan, Y.; Chotivanich, K.; et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 2015, 520, 683–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toker, A. Protein kinases as mediators of phosphoinositide 3-kinase signaling. Mol. Pharmacol. 2000, 57, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Luis, S.; Ballesteros, J.; Gutiérrez, M. Antibacterial Constituents from the Octocoral-Associated bacterium Pseudoalteromonas sp. Rev. Latinoamer. Quím. 2011, 39, 75–83. [Google Scholar]
- Martínez-Luis, S.; Gómez, J.F.; Spadafora, C.; Guzmán, H.M.; Gutiérrez, M. Antitrypanosomal Alkaloids from the Marine Bacterium Bacillus pumilus. Molecules 2012, 17, 11146–11155. [Google Scholar] [CrossRef] [PubMed]
- Boya, C.A.; Herrera, L.; Guzman, H.M.; Gutiérrez, M. Antiplasmodial Activity of Bacilosarcin A Isolated from the Octocoral-associated Bacterium Bacillus sp. Collected in Panama. J. Pharm. Bioallied Sci. 2012, 4, 66–69. [Google Scholar] [PubMed]
- Boya, C.A.; Fernández-Marín, H.; Mejía, L.C.; Spadafora, C.; Dorrestein, P.C.; Gutiérrez, M. Imaging mass spectrometry and MS/MS molecular networking reveals chemical interactions among cuticular bacteria and pathogenic fungi associated with fungus-growing ants. Sci. Rep. 2017, 7, 5604. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Kapono, C.A.; Lim, Y.W.; Koyama, N.; Vermeij, M.J.A.; Conrad, D.; Rohwer, F.; Dorrestein, P.C. Mass spectral similarity for untargeted metabolomics data analysis of complex mixtures. Int. J. Mass Spectrom. 2015, 377, 719–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, J.D.; Diggs, C.L.; Hines, F.A.; Desjardins, R.E. Culture of human malaria parasites Plasmodium falciparum. Nature 1976, 263, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Almanza, A.; Herrera, L.; Tayler, N.; Coronado, L.; Spadafora, C. Automated Synchronization of P. falciparum using a Temperature Cycling Incubator. Curr. Trends Biotechnol. Pharm. 2011, 5, 1130–1133. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Corbett, Y.; Herrera, L.; Gonzalez, J.; Cubilla, L.; Capson, T.L.; Coley, P.D.; Kursar, T.A.; Romero, L.I.; Ortega-Barria, E. A novel DNA-based microfluorimetric method to evaluate antimalaria drug activity. Am. J. Trop. Med. Hyg. 2004, 70, 119–124. [Google Scholar] [PubMed]
- Costa-Lotufo, L.V.; Khan, M.T.H.; Ather, A.; Wilke, D.V.; Jimenez, P.C.; Pessoa, C.; De Moraes, M.E.A.; De Moraes, M.O. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J. Ethnopharmacol. 2005, 99, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, D.; Coetzer, T.L. Turning up the heat: Heat stress induces markers of programmed cell death in Plasmodium falciparum in vitro. Cell Death Dis. 2013, 4, e971. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, W.; Bartosz, G. 2,7-dichlorofluorescin oxidation and reactive oxygen species: What does it measure? Cell Biol. Int. 2000, 24, 757–760. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds (1) and (2) are available from the authors. |




| Compound | Parasite Stage | IC50 (μM) | SEM |
|---|---|---|---|
| Pumilacidin A | schizont | 8.34 | 0.97 |
| ring | 15.44 | 3.9 | |
| Pumilacidin C | schizont | 7.75 | 1.74 |
| ring | 19.59 | 4.4 |
| Compound | IC50 (μM) | SEM |
|---|---|---|
| Pumilacidin A | 28.42 | 9.86 |
| Pumilacidin C | 26.07 | 10.10 |
| Inhibitor | Nomenclature Used | Target |
|---|---|---|
| 3-Methyladenine (3MA) | Inhibitor 1 | Phosphatidylinositol 3-kinases (PI3Ks) I and III |
| LY29400 | Inhibitor 2 | PI3-kinase I (PI3K) |
| Protein kinase C (PKC) inhibitor (bisindolylmaleimide I) | Inhibitor 3 | PKC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Mendoza, D.; Coronado, L.M.; Pineda, L.M.; Guzmán, H.M.; Dorrestein, P.C.; Spadafora, C.; Gutiérrez, M. Pumilacidins from the Octocoral-Associated Bacillus sp. DT001 Display Anti-Proliferative Effects in Plasmodium falciparum. Molecules 2018, 23, 2179. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092179
Torres-Mendoza D, Coronado LM, Pineda LM, Guzmán HM, Dorrestein PC, Spadafora C, Gutiérrez M. Pumilacidins from the Octocoral-Associated Bacillus sp. DT001 Display Anti-Proliferative Effects in Plasmodium falciparum. Molecules. 2018; 23(9):2179. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092179
Chicago/Turabian StyleTorres-Mendoza, Daniel, Lorena M. Coronado, Laura M. Pineda, Héctor M. Guzmán, Pieter C. Dorrestein, Carmenza Spadafora, and Marcelino Gutiérrez. 2018. "Pumilacidins from the Octocoral-Associated Bacillus sp. DT001 Display Anti-Proliferative Effects in Plasmodium falciparum" Molecules 23, no. 9: 2179. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092179





