Actinofuranones D-I from a Lichen-Associated Actinomycetes, Streptomyces gramineus, and Their Anti-Inflammatory Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation of Actinofuranones D-I
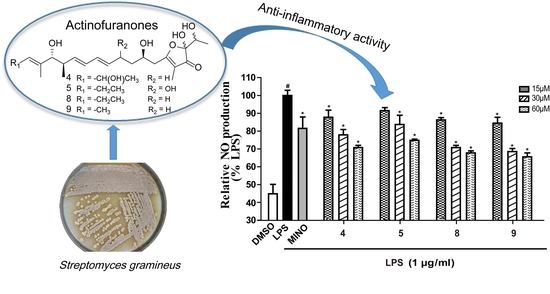
2.2. Cell Viability and Effects of Compounds on the Production of NO in LPS-Induced RAW 264.7 Cells
2.3. Compounds 4, 5, 8, and 9 Attenuated LPS-Induced iNOS Expression in RAW 264.7 Cells
2.4. Compounds 4, 5, 8, and 9 Suppressed Release of IL-6 and TNF-α in LPS-Induced RAW 264.7 Cells
3. Materials and Methods
3.1. General Information
3.2. Microbial Material
3.3. Fermentation, Extraction and Isolation
3.4. Cell Culture and Vability Assay
3.5. Nitric Oxide (NO) Production Assay
3.6. Western Blot
3.7. Measurement of IL-6 and TNF-α by an Enzyme-Linked Immunosorbent Assay (ELISA)
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bates, S.T.; Cropsey, G.W.; Caporaso, J.G.; Knight, R.; Fierer, N. Bacterial communities associated with the lichen symbiosis. Appl. Environ. Microbiol. 2011, 77, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Joshi, G.P.; Rawat, M.S.M. Lichens as a potential natural source of bioactive compounds: A review. Phytochem. Rev. 2010, 9, 303–314. [Google Scholar] [CrossRef]
- Kellogg, J.J.; Raja, H.A. Endolichenic fungi: A new source of rich bioactive secondary metabolites on the horizon. Phytochem. Rev. 2017, 16, 271–293. [Google Scholar] [CrossRef]
- Davies, J.; Wang, H.; Taylor, T.; Warabi, K.; Huang, X.; Andersen, R.J. Uncialamycin, a new enediyne antibiotic. Org. Lett. 2005, 7, 5233–5236. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Davies, J.; Patrick, B.O.; Bottriell, H.; Tarling, T.; Roberge, M.; Andersen, R.J. Cladoniamides A−G, tryptophan-derived alkaloids produced in culture by Streptomyces uncialis. Org. Lett. 2008, 10, 3501–3504. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, K.; Takagi, M.; Yamamura, H.; Hayakawa, M.; Shin-ya, K. A new angucycline and a new butenolide isolated from lichen-derived Streptomyces spp. J. Antibiot. 2010, 63, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Cheenpracha, S.; Vidor, N.B.; Yoshida, W.Y.; Davies, J.; Chang, L.C. Coumabiocins A−F, aminocoumarins from an organic extract of Streptomyces sp. L-4-4. J. Nat. Prod. 2010, 73, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, Y.; Lei, H.; Chen, X.; Ma, Q.; Han, L.; Huang, X. Four new nanaomycins produced by Streptomyces hebeiensis derived from lichen. Chem. Biodivers. 2017, 14, e1700057. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, Y.; Wang, X.; Chen, D.; Chen, X.; Wang, L.; Han, L.; Huang, X.; Jiang, C. Diversity, antimicrobial activity, and biosynthetic potential of cultivable actinomycetes associated with lichen symbiosis. Microb. Ecol. 2017, 74, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Tsukamoto, H.; Izumikawa, M.; Hosoya, T.; Kagaya, N.; Takagi, M.; Yamamura, H.; Hayakawa, M.; Shin-Ya, K.; Doi, T. Total synthesis and structure determination of JBIR-108-a 2-hydroxy-2-(1-hydroxyethyl)-2,3-dihydro-3(2H)-furanone isolated from Streptomyces gramineus IR087Pi-4. J. Org. Chem. 2015, 80, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Mcalpine, J.B.; Sørensen, D.; Aouidate, M.; Piraee, M.; Alarco, A.M.; Omura, S.; Shiomi, K.; Farnet, C.M.; Zazopoulos, E. Isolation and identification of three new 5-alkenyl-3,3 (2H)-furanones from two Streptomyces species using a genomic screening approach. J. Antibiot. 2006, 59, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kwon, H.C.; Williams, P.G.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Actinofuranones A and B, polyketides from a marine-derived bacterium related to the genus Streptomyces (Actinomycetales). J. Nat. Prod. 2006, 69, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Shin, J.H. Actinofuranone C, a new 3-furanone-bearing polyketide from a dung beetle-associated bacterium. Nat. Prod. Sci. 2013, 19, 71–75. [Google Scholar]
- Indananda, C.; Igarashi, Y.; Ikeda, M.; Oikawa, T.; Thamchaipenet, A. Linfuranone A, a new polyketide from plant-derived Microbispora sp. GMKU 363. J. Antibiot. 2013, 66, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Faulkner, D.J. Metabolites of the pulmonate Siphonaria lessoni. J. Org. Chem. 1984, 49, 2506–2508. [Google Scholar] [CrossRef]
- Beukes, D.R.; Davies-Coleman, M.T. Novel polypropionates from the South African marine mollusc Siphonaria capensis. Tetrahedron 1999, 55, 4051–4056. [Google Scholar] [CrossRef]
- Bromley, C.L.; Popplewell, W.L.; Pinchuck, S.C.; Hodgson, A.N.; Davies-Coleman, M.T. Polypropionates from the South African marine mollusk Siphonaria oculus. J. Nat. Prod. 2012, 75, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Cutignano, A.; Giordano, A.; Coll, A.D.; Cimino, G. Biosynthesis of aglajnes, polypropionate allomones of the opisthobranch mollusc Bulla striata. Tetrahedron Lett. 2004, 45, 6847–6850. [Google Scholar] [CrossRef]
- Kuroda, K.; Yoshida, M.; Uosaki, Y.; Ando, K.; Kawamoto, I.; Oishi, E.; Onuma, H.; Yamada, K.; Matsuda, Y. AS-183, a novel inhibitor of acyl-CoA: Cholesterol acyltransferase produced by Scedosporium sp. SPC-15549. J. Antibiot. 1993, 46, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Kunze, B.; Reichenbach, H.; Müller, R.; Höfle, G. Aurafuron A and B, new bioactive polyketides from Stigmatella aurantiaca and Archangium gephyra (myxobacteria). J. Antibiot. 2005, 58, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, O.; Kalesse, M. The total synthesis of (−)-aurafuron A. Org. Lett. 2012, 14, 3064–3067. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.C.; Kim, J.C.; Song, S.E.; Suh, S.J.; Seo, C.S.; Kim, Y.K.; Jin, M.; Yang, J.H.; Son, J.K.; Jahng, Y.; et al. Saucerneol D, a naturally occurring sesquilignan, inhibits LPS-induced iNOS expression in RAW 264. 7 cells by blocking NF-κB and MAPK activation. Int. Immunopharmacol. 2008, 8, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Lai, C.S.; Wang, J.M.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Differential inhibitory effects of inotilone on inflammatory mediators, inducible nitric oxide synthase and cyclooxygenase-2, in LPS-stimulated murine macrophage. Mol. Nutr. Food Res. 2009, 53, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Jeong, H.J. Rheosmin, a naturally occurring phenolic compound inhibits LPS-induced iNOS and COX-2 expression in RAW264. 7 cells by blocking NF-κB activation pathway. Food Chem. Toxicol. 2010, 48, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Muñoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Thomson, I.C.; Fon, J.; Uylaki, W.; Cummins, A.G.; Barry, S. Cells, cytokines and inflammatory bowel disease: A clinical perspective. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 703–716. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–9 are available from the authors. |






| No. | 1 | 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| δCa, Type | δHa, [J in Hz] | δCb, Type | δHb, [J in Hz] | δCa, Type | δHa, [J in Hz] | δCb, Type | δHb, [J in Hz] | |
| 1 | 68.2 (69.3), CH | 3.69 (3.74), m | 70.4 (71.0), CH | 3.90, m | 68.2 (69.3), CH | 3.69 (3.74), m | 70.3 (70.9), CH | 3.90, m |
| 2 | 104.2 (104.6), C | 105.6 (105.5), C | 104.1 (104.6), C | 105.5 (105.6), C | ||||
| 3 | 202.9 (201.6), C | 205.9 (204.4), C | 202.9 (201.6), C | 205.7 (204.2), C | ||||
| 4 | 109.8 (110.4), C | 112.2 (112.6), C | 109.8 (110.4), C | 112.0 (112.5), C | ||||
| 5 | 185.4 (185.1), C | 188.6 (188.2), C | 185.4 (185.1), C | 188.4 (187.9), C | ||||
| 6 | 37.7 (37.4), CH2 | 2.58 (2.63), d [6.8] 2.58 (2.56), d [6.8] | 38.4 (38.2), CH2 | 2.71 (2.76), m 2.71 (2.70), m | 37.6 (37.4), CH2 | 2.58 (2.64), d [7.0] 2.58 (2.55), d [7.0] | 38.1 (38.3), CH2 | 2.70 (2.75), d [7.7] 2.70 (2.69), d [7.7] |
| 7 | 67.9 (67.7), CH | 3.81 (3.86), m | 67.9 (67.7), CH | 3.98 (4.05), m | 67.9 (67.7), CH | 3.81 (3.86), m | 69.6 (69.4), CH | 3.98 (4.05), m |
| 8 | 36.5 (36.7), CH2 | 1.60, m; 1.46, m | 38.2 (38.1), CH2 | 1.64, m | 36.6 (36.7), CH2 | 1.56, m; 1.46, m | 38.1 (38.0), CH2 | 1.70, m; 1.62, m |
| 9 | 28.6, CH2 | 2.17, m; 2.07, m | 29.9, CH2 | 2.26, m; 2.18, m | 28.6, CH2 | 2.16, m; 2.07, m | 29.7, CH2 | 2.25, m; 2.17, m |
| 10 | 132.0, CH | 5.55, dt [14.2, 6.8] | 132.2, CH | 5.61, m | 132.1, CH | 5.55, dt [14.6, 7.0] | 132.5, CH | 5.59, dt [14.5, 7.0] |
| 11 | 131.1, CH | 5.97, m | 132.7, CH | 6.08, m | 131.1, CH | 5.93, m | 132.5, CH | 6.05, m |
| 12 | 129.7, CH | 5.94, m | 132.2, CH | 6.06, m | 129.7, CH | 5.90, m | 131.8, CH | 6.03, m |
| 13 | 136.3, CH | 5.61, dd [14.5, 7.3] | 136.3, CH | 5.58, m | 135.4, CH | 5.55, dd [14.6, 7.6] | 135.6, CH | 5.55, dd [14.5, 8.0] |
| 14 | 40.4, CH | 2.19, sextet [7.0] | 41.7, CH | 2.31, m | 40.0, CH | 2.25, sextet [7.0] | 41.5, CH | 2.35, sextet [7.0] |
| 15 | 81.0, CH | 3.50, dd, [7.8, 4.7] | 83.3, CH | 3.65, d, [8.5] | 80.0, CH | 3.56, brt, [5.6] | 82.3, CH | 3.70, d, [7.0] |
| 16 | 136.7, C | 139.2, C | 136.9, C | 139.2, C | ||||
| 17 | 131.4, CH | 5.17, d [8.7] | 132.0, CH | 5.30, d [8.7] | 130.2, CH | 5.22, d [8.6] | 130.9, CH | 5.33, d [8.7] |
| 18 | 68.3, CH | 4.10, m | 69.8 (69.5), CH | 4.27, m | 68.3, CH | 4.08, m | 69.9, CH | 4.26, m |
| 19 | 31.0, CH2 | 1.42, m; 1.30, m | 31.6, CH2 | 1.57, m; 1.42, m | 31.0, CH2 | 1.43, m; 1.28, m | 31.5, CH2 | 1.57, m; 1.43, m |
| 20 | 10.1, CH3 | 0.79, t [7.0] | 10.2, CH3 | 0.88, t [7.2] | 10.1, CH3 | 0.78, t [7.3] | 10.1, CH3 | 0.88, t [7.2] |
| 21 | 17.1 (16.5), CH3 | 1.13 (1.04), d [6.4] | 16.8 (16.4), CH3 | 1.29 (1.17), d [6.5] | 17.1 (16.5), CH3 | 1.13 (1.04), d [6.4] | 16.7 (16.3), CH3 | 1.29 (1.17), d [6.4] |
| 22 | 6.0, CH3 | 1.53 (1.54), s | 5.8, CH3 | 1.65 (1.64), s | 6.0, CH3 | 1.53, s | 5.7, CH3 | 1.65 (1.64), s |
| 23 | 17.4, CH3 | 0.78, d [7.0] | 18.0, CH3 | 0.85, d [7.2] | 17.9, CH3 | 0.88, d [6.6] | 18.1, CH3 | 0.95, d [7.2] |
| 24 | 11.7, CH3 | 1.52, s | 11.7, CH3 | 1.65, s | 13.0, CH3 | 1.50, s | 12.7, CH3 | 1.63, s |
| 1-OH | 4.67 (4.86), d, [6.4] | 4.66 (4.85), d [6.4] | ||||||
| 2-OH | 7.25 (7.32), s | 7.23 (7.35), s | ||||||
| 7-OH | 4.70 (4.80), d [5.1] | 4.68 (4.78), d [5.3] | ||||||
| 15-OH | 4.61, d [4.6] | 4.59, d [4.7] | ||||||
| 18-OH | 4.45, d [4.8] | 4.37, d [4.5] | ||||||
| No. | 3 | 4 | ||
|---|---|---|---|---|
| δC, Type | δH, [J in Hz] | δC, Type | δH, [J in Hz] | |
| 1 | 68.3 (69.3), CH | 3.70 (3.75), m | 68.2 (69.3), CH | 3.69 (3.74), m |
| 2 | 104.1 (104.5), C | 104.1 (104.6), C | ||
| 3 | 202.8 (201.6), C | 202.9 (201.6), C | ||
| 4 | 109.8 (110.3), C | 109.8 (110.4), C | ||
| 5 | 185.1 (185.4), C | 185.4 (185.1), C | ||
| 6 | 37.8 (37.4), CH2 | 2.58 (2.62), m 2.58 (2.58), m | 37.6 (37.4), CH2 | 2.58 (2.63), d [6.5] 2.58 (2.56), d [6.5] |
| 7 | 67.9 (67.7), CH | 3.81 (3.86), m | 67.9 (67.7), CH | 3.81 (3.86), m |
| 8 | 36.6 (36.7), CH2 | 1.59, m; 1.45, m | 36.6 (36.7), CH2 | 1.57, m; 1.45, m |
| 9 | 28.6, CH2 | 2.16, m; 2.08, m | 28.6, CH2 | 2.16, m; 2.08, m |
| 10 | 131.9, CH | 5.54, dt [14.0, 6.6] | 132.0, CH | 5.54, dt [13.6, 6.7] |
| 11 | 131.1, CH | 5.96, m | 131.1, CH | 5.96, m |
| 12 | 129.6, CH | 5.92, m | 129.7, CH | 5.92, m |
| 13 | 136.1, CH | 5.58, dd [14.8, 7.5] | 135.8, CH | 5.56, dd [15.1, 7.5] |
| 14 | 40.4, CH | 2.21, sextet [7.3] | 40.4, CH | 2.22, sextet [7.0] |
| 15 | 81.0, CH | 3.53, dd [7.7, 3.0] | 80.2, CH | 3.52, dd, [7.0, 4.8] |
| 16 | 138.2, C | 135.8, C | ||
| 17 | 123.1, CH | 5.28, t [7.3] | 132.0, CH | 5.25, d [8.1] |
| 18 | 37.8 CH2 | 2.08, m; 1.98, m | 63.0, CH | 4.35, m |
| 19 | 66.6, CH | 3.58, q [6.0] | 24.4, CH3 | 1.05, d [6.2] |
| 20 | 23.4, CH3 | 1.00, d [6.2] | 17.1 (16.5), CH3 | 1.13 (1.04), d [6.4] |
| 21 | 17.1 (16.5), CH3 | 1.14 (1.04), d [6.4] | 6.0, CH3 | 1.53, s |
| 22 | 6.0, CH3 | 1.53, s | 17.7, CH3 | 0.84, d [6.8] |
| 23 | 17.6, CH3 | 0.79, d [6.9] | 12.4 (12.9), CH3 | 1.50, s |
| 24 | 12.0, CH3 | 1.49, s | ||
| 1-OH | 4.68 (4.90), brs | 4.67 (4.85), d [6.4] | ||
| 2-OH | 7.33, s | 7.23 (7.35), s | ||
| 7-OH | 4.72 (4.79), brs | 4.69 (4.78), d [5.4] | ||
| 15-OH | 4.52, d [3.4] | |||
| 18-OH | 4.57, d [4.6] | |||
| 19-OH | 4.40, brs | 4.44, d [4.4] | ||
| No. | 5 | 6 | 7 | |||
|---|---|---|---|---|---|---|
| δC, Type | δH, [J in Hz] | δC, Type | δH, [J in Hz] | δC, Type | δH, [J in Hz] | |
| 1 | 68.3 (69.3), CH | 3.69 (3.74), m | 68.2 (69.3), CH | 3.69 (3.74), m | 68.4 (69.4), CH | 3.70 (3.74), m |
| 2 | 104.2 (104.6), C | 104.2 (104.6), C | 104.3 (104.7), C | |||
| 3 | 202.9 (201.6), C | 202.9 (201.6), C | 203.0 (201.7), C | |||
| 4 | 109.9 (110.5), C | 109.8 (110.4), C | 109.9 (110.5), C | |||
| 5 | 185.3 (185.1), C | 185.4 (185.1), C | 185.5 (185.2), C | |||
| 6 | 37.7 (37.4), CH2 | 2.62, (2.62, 2.58), m | 37.6 (37.4), CH2 | 2.56 (2.62, 2.56), m | 37.7 (37.5), CH2 | 2.57 (2.64, 2.56), d [7.0] |
| 7 | 66.5 (66.3), CH | 3.90 (3.96), m | 67.9 (67.7), CH | 3.81 (3.86), m | 67.8 (68.0), CH | 3.81 (3.86), m |
| 8 | 44.5 (44.6), CH2 | 1.61 (1.59, 1.46), m | 36.5 (36.6), CH2 | 1.59, m; 1.46, m | 36.7 (36.8), CH2 | 1.57 (1.56), m; 1.45 (1.46), m |
| 9 | 69.2 (69.1), CH | 4.16, m | 28.7, CH2 | 2.18, m; 2.09, m | 28.7, CH2 | 2.18, m; 2.07, m |
| 10 | 135.3, CH | 5.54, dt [15.1, 6.2] | 132.9 (132.8), CH | 5.59, dt [15.1, 7.0] | 132.1, CH | 5.55 (5.53), dt [14.8, 7.0] |
| 11 | 130.1, CH | 6.10, m | 130.7, CH | 6.08, dd, [15.1, 10.5] | 131.3, CH | 5.95, m |
| 12 | 129.2, CH | 5.96, dd [15.2, 10.4] | 127.3 (127.2), CH | 5.99, dd [15.1, 10.5] | 129.7, CH | 5.95, m |
| 13 | 137.8, CH | 5.67, dd [15.3, 7.4] | 138.5 (138.6), CH | 5.65, brd [15.1] | 136.3, CH | 5.59 (5.55), dd [15.0, 7.5] |
| 14 | 40.4, CH | 2.23, sextet [7.0] | 74.7, C | 40.0, CH | 2.21, sextet [7.0] | |
| 15 | 80.8, CH | 3.52, dd, [7.5, 3.8] | 82.6, CH | 3.63, d, [4.4] | 81.1, CH | 3.53, dd [7.8, 4.4] |
| 16 | 136.5, C | 135.4, C | 137.4, C | |||
| 17 | 127.7, CH | 5.25, t [7.5] | 129.3, CH | 5.23, t [7.0] | 126.0, CH | 5.24, t [7.3] |
| 18 | 20.6, CH2 | 1.97, quint [7.5] | 20.7, CH2 | 1.95, quint [7.3] | 29.5, CH2 | 1.94, q [7.3] |
| 19 | 14.5, CH3 | 0.90, t [7.5] | 14.4, CH3 | 0.89, t [7.5] | 22.8, CH2 | 1.32 m |
| 20 | 17.1, CH3 | 1.14 (1.04), d [7.5] | 17.1 (16.5), CH3 | 1.13 (1.04), d [6.4] | 14.1, CH3 | 0.85, t [7.4] |
| 21 | 6.0, CH3 | 1.53, s | 6.0, CH3 | 1.53, s | 17.2 (16.6), CH3 | 1.14 (1.04), d [6.4] |
| 22 | 17.5, CH3 | 0.80, d [7.0] | 25.4 (25.5), CH3 | 1.06, s | 6.1, CH3 | 1.54, s |
| 23 | 11.6, CH3 | 1.49, s | 13.8, CH3 | 1.53, s | 17.7, CH3 | 0.80, d [6.9] |
| 24 | 11.9, CH3 | 1.49, s | ||||
| 1-OH | 4.67 (4.85), brs | 4.66 (4.85), d [6.4] | 4.66 (4.84), d [4.5] | |||
| 2-OH | 7.25 (7.35), s | 7.24 (7.35), s | 7.23 (7.34), s | |||
| 7-OH | 4.72 (4.81), m | 4.69 (4.78), d [5.3] | 4.68 (4.78), brs | |||
| 9-OH | 4.76 (4.80), brs | |||||
| 14-OH | 4.17, s | |||||
| 15-OH | 4.54, d [3.8] | 4.62, d [4.5] | 4.52, d [3.6] | |||
| 18-OH | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Cao, B.; Liu, C.; Guan, P.; Mu, Y.; Jiang, Y.; Han, L.; Huang, X. Actinofuranones D-I from a Lichen-Associated Actinomycetes, Streptomyces gramineus, and Their Anti-Inflammatory Effects. Molecules 2018, 23, 2393. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092393
Ma J, Cao B, Liu C, Guan P, Mu Y, Jiang Y, Han L, Huang X. Actinofuranones D-I from a Lichen-Associated Actinomycetes, Streptomyces gramineus, and Their Anti-Inflammatory Effects. Molecules. 2018; 23(9):2393. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092393
Chicago/Turabian StyleMa, Jian, Bixuan Cao, Chengbin Liu, Peipei Guan, Yu Mu, Yi Jiang, Li Han, and Xueshi Huang. 2018. "Actinofuranones D-I from a Lichen-Associated Actinomycetes, Streptomyces gramineus, and Their Anti-Inflammatory Effects" Molecules 23, no. 9: 2393. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092393