Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. on Obesity
Abstract
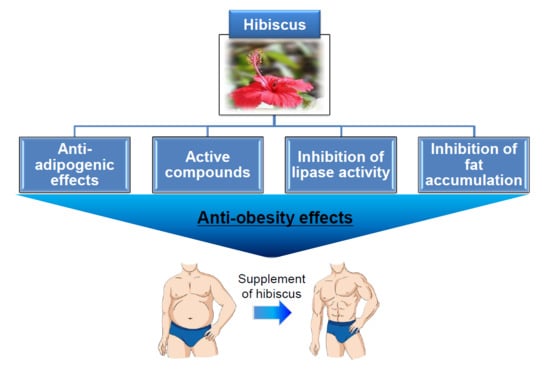
:1. Introduction
2. Natural Bioactive Compounds in H. sabdariffa
3. Effect of H. sabdariffa Extract (HSE) on Obesity
3.1. Effect of HSE on Body Weight
3.2. Effect of HSE on Lipid Accumulation, Cholesterol Metabolism and Plasma Parameters
3.3. Inhibitory Effect of HSE on Pancreatic Lipase
3.4. Effect of HSE on Adipocyte Differentiation (Adipogenesis)
4. Toxicity of H. sabdariffa
5. Conclusion
Author Contributions
Conflicts of Interest
Abbreviations
| Adipose tissue | AT |
| Epigallocatechin gallate | EGCG |
| Free fatty acid | FFA |
| High-density lipoproteins | HDL |
| Hibiscus sabdariffa extract | HSE |
| Interleukin-1 | IL-1 |
| Low-density lipoproteins | LDL |
| Messenger RNA | mRNA |
| Metabolic syndrome | MS |
| Not specified | NS |
| Peroxisome Proliferator-activated Receptor γ | PPARγ |
| Sterol regulatory element-binding protein | SREBP-1c |
| Tumor necrosis factor α | TNF-α |
References
- Laura Segal, J.; Martin, A. The State of Obesity: Better Policies for a Healthier America 2016; Robert Wood Johnson Foundation: Princeton, NJ, USA, 2016. [Google Scholar]
- Lee, S.G.; Lee, Y.J.; Jang, M.H.; Kwon, T.R.; Nam, J.O. Panax ginseng Leaf Extracts Exert Anti-Obesity Effects in High-Fat Diet-Induced Obese Rats. Nutrients 2017, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, S.; Mohanam, L.; Alwin, D.; Prabhu, V. Effect of combination therapy of melatonin and orlistat on high fat diet induced changes in lipid profiles and liver function parameters in serum of rats. Obes. Med. 2016, 2, 41–45. [Google Scholar] [CrossRef]
- Sharmila, B.G.; Kumar, G.; Rajasekara, P.M. Cholesterol lowering activity of the aqueous fruit extract of Trichosanthes dioica Roxb. in normal and streptozotocin diabetic rats. J. Clin. Diagn. Res. 2007, 1, 561–569. [Google Scholar]
- Abdul Rahman, H.; Saari, N.; Abas, F.; Ismail, A.; Mumtaz, M.W.; Abdul Hamid, A. Anti-obesity and antioxidant activities of selected medicinal plants and phytochemical profiling of bioactive compounds. Int. J. Food Prop. 2017, 20, 2616–2629. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. A natural solution for obesity: Bioactives for the prevention and treatment of weight gain. A review. Nutr. Neurosci. 2015, 18, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Tung, Y.C.; Yang, G.; Li, S.; Ho, C.T. Molecular mechanisms of the anti-obesity effect of bioactive compounds in tea and coffee. Food Funct. 2016, 7, 4481–4491. [Google Scholar] [CrossRef]
- Kao, E.S.; Yang, M.Y.; Hung, C.H.; Huang, C.N.; Wang, C.J. Polyphenolic extract from Hibiscus sabdariffa reduces body fat by inhibiting hepatic lipogenesis and preadipocyte adipogenesis. Food Funct. 2016, 7, 171–182. [Google Scholar] [CrossRef]
- Alarcon-Aguilar, F.J.; Zamilpa, A.; Perez-Garcia, M.D.; Almanza-Perez, J.C.; Romero-Nunez, E.; Campos-Sepulveda, E.A.; Vazquez-Carrillo, L.I.; Roman-Ramos, R. Effect of Hibiscus sabdariffa on obesity in MSG mice. J. Ethnopharmacol. 2007, 114, 66–71. [Google Scholar] [CrossRef]
- Kim, J.K.; So, H.; Youn, M.J.; Kim, H.J.; Kim, Y.; Park, C.; Kim, S.J.; Ha, Y.A.; Chai, K.Y.; Kim, S.M.; et al. Hibiscus sabdariffa L. water extract inhibits the adipocyte differentiation through the PI3-K and MAPK pathway. J. Ethnopharmacol. 2007, 114, 260–267. [Google Scholar] [CrossRef]
- Huang, T.W.; Chang, C.L.; Kao, E.S.; Lin, J.H. Effect of Hibiscus sabdariffa extract on high fat diet-induced obesity and liver damage in hamsters. Food Nutr. Res. 2015, 59, 29018. [Google Scholar] [CrossRef]
- Carvajal-Zarrabal, O.; Hayward-Jones, P.M.; Orta-Flores, Z.; Nolasco-Hipolito, C.; Barradas-Dermitz, D.M.; Aguilar-Uscanga, M.G.; Pedroza-Hernandez, M.F. Effect of Hibiscus sabdariffa L. dried calyx ethanol extract on fat absorption-excretion and body weight implication in rats. J. Biomed. Biotechnol. 2009, 2009, 394592. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Cahlikova, L.; Opletal, L.; Karaca, T.; Manoj, P.; Ramkumar, A.; Al Suleimani, Y.M.; Al Za’abi, M.; Nemmar, A.; Chocholousova-Havlikova, L.; Locarek, M.; Siatka, T.; Blunden, G. Effect of aqueous extract and anthocyanins of calyces of Hibiscus sabdariffa (Malvaceae) in rats with adenine-induced chronic kidney disease. J. Pharm. Pharmacol. 2017, 69, 1219–1229. [Google Scholar] [CrossRef]
- Lin, H.H.; Chen, J.H.; Wang, C.J. Chemopreventive properties and molecular mechanisms of the bioactive compounds in Hibiscus sabdariffa Linne. Curr. Med. Chem. 2011, 18, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Moyano, G.; Sáyago-Ayerdi, S.G.; Largo, C.; Caz, V.; Santamaria, M.; Tabernero, M. Potential use of dietary fibre from Hibiscus sabdariffa and Agave tequilana in obesity management. J. Funct. Food 2016, 21, 1–9. [Google Scholar] [CrossRef]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Tech. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Perez-Torres, I.; Ruiz-Ramirez, A.; Banos, G.; El-Hafidi, M. Hibiscus sabdariffa Linnaeus (Malvaceae), curcumin and resveratrol as alternative medicinal agents against metabolic syndrome. Cardiovasc. Hematol. Agents Med. Chem. 2013, 11, 25–37. [Google Scholar] [CrossRef]
- Tseng, T.H.; Kao, T.W.; Chu, C.Y.; Chou, F.P.; Lin, W.L.; Wang, C.J. Induction of apoptosis by hibiscus protocatechuic acid in human leukemia cells via reduction of retinoblastoma (RB) phosphorylation and Bcl-2 expression. Biochem. Pharmacol. 2000, 60, 307–315. [Google Scholar] [CrossRef]
- Darwish, R.M.; Aburjai, T.; Al-Khalil, S.; Mahafzah, A. Screening of antibiotic resistant inhibitors from local plant materials against two different strains of Staphylococcus aureus. J Ethnopharmacol. 2002, 79, 359–364. [Google Scholar] [CrossRef]
- Amos, S.; Binda, L.; Chindo, B.; Tseja, A.; Odutola, A.; Wambebe, C.; Gamaniel, K. Neuropharmacological effects of Hibiscus sabdariffa aqueous extract. Pharm. Biol. 2003, 41, 325–329. [Google Scholar] [CrossRef]
- Odigie, I.P.; Ettarh, R.R.; Adigun, S.A. Chronic administration of aqueous extract of Hibiscus sabdariffa attenuates hypertension and reverses cardiac hypertrophy in 2K-1C hypertensive rats. J. Ethnopharmacol. 2003, 86, 181–185. [Google Scholar] [CrossRef]
- Herrera-Arellano, A.; Miranda-Sanchez, J.; Avila-Castro, P.; Herrera-Alvarez, S.; Jimenez-Ferrer, J.E.; Zamilpa, A.; Roman-Ramos, R.; Ponce-Monter, H.; Tortoriello, J. Clinical effects produced by a standardized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Med. 2007, 73, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Hirunpanich, V.; Utaipat, A.; Morales, N.P.; Bunyapraphatsara, N.; Sato, H.; Herunsale, A.; Suthisisang, C. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J. Ethnopharmacol. 2006, 103, 252–260. [Google Scholar] [CrossRef]
- Ali, B.H.; Wabel, N.A.; Blunden, G. Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L.: A review. Phytother. Res. 2005, 19, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Mungole, A.; Chaturvedi, A. Hibiscus sabdariffa L a rich source of secondary metabolites. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 83–87. [Google Scholar]
- Wahabi, H.A.; Alansary, L.A.; Al-Sabban, A.H.; Glasziuo, P. The effectiveness of Hibiscus sabdariffa in the treatment of hypertension: A systematic review. Phytomedicine 2010, 17, 83–86. [Google Scholar] [CrossRef]
- Carvajal-Zarrabal, O.; Barradas-Dermitz, D.M.; Orta-Flores, Z.; Hayward-Jones, P.M.; Nolasco-Hipólito, C.; Aguilar-Uscanga, M.G.; Miranda-Medina, A.; Bujang, K.B. Hibiscus sabdariffa L., roselle calyx, from ethnobotany to pharmacology. J Exp. Pharm. 2012, 4, 25. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Lamm, M.G.; Funk, J.L.; Ritenbaugh, C. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: A comprehensive review of animal and human studies. Fitoterapia 2013, 85, 84–94. [Google Scholar] [CrossRef]
- Serban, C.; Sahebkar, A.; Ursoniu, S.; Andrica, F.; Banach, M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: A systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2015, 33, 1119–1127. [Google Scholar] [CrossRef]
- Walton, R.J.; Whitten, D.L.; Hawrelak, J.A. The efficacy of Hibiscus sabdariffa (rosella) in essential hypertension: A systematic review of clinical trials. Aust. J. Herb. Med. 2016, 28, 48. [Google Scholar]
- Singh, P.; Khan, M.; Hailemariam, H. Nutritional and Health Importance of Hibiscus Sabdariffa: A Review and Indication for Research Needs. J. Nutr. Health Food Eng. 2017, 6, 1–4. [Google Scholar]
- Ismail, A.; Ikram, E.H.K.; Nazri, H.S.M. Roselle (Hibiscus sabdariffa L.) seeds-nutritional composition, protein quality and health benefits. Food 2008, 2, 1–16. [Google Scholar]
- Herranz-Lopez, M.; Olivares-Vicente, M.; Encinar, J.A.; Barrajon-Catalan, E.; Segura-Carretero, A.; Joven, J.; Micol, V. Multi-Targeted Molecular Effects of Hibiscus sabdariffa Polyphenols: An Opportunity for a Global Approach to Obesity. Nutrients 2017, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.; Wong, S.Y.; Chong, N.J. Effects of Hibiscus sabdariffa L. on serum lipids: A systematic review and meta-analysis. J. Ethnopharmacol. 2013, 150, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, A.; Siem, H.; Bjertness, E.; Meltzer, M.; Flaten, T.; Holmsen, E. Bioactive compounds in plants–benefits and risks for man and animals. In Proceedings of the Symposium Held at The Norwegian Academy of Science and Letters, Oslo, Norway, 13–14 November 2008. [Google Scholar]
- Kumar, G.; Sharmila Banu, G.; Murugesan, A.G. Attenuation of Helicteres isora L. bark extracts on streptozotocin-induced alterations in glycogen and carbohydrate metabolism in albino rats. Hum. Exp. Toxicol. 2009, 28, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Ramasamy, M.; Gani, S.B. Antihyperlipideamic effect of Solanum trilobatum L. leaves extract on streptozotocin induced diabetic rats. Asian J. Biomed. Pharm. Sci. 2013, 3, 51–56. [Google Scholar]
- Igho, O.; Kang, H.S.; Rachel, P.; Barbara, W.; Edzard, E. The use of Garcinia extract (hydroxycitric acid) as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. J. Obes. 2010, 2011, 1–9. [Google Scholar]
- Heymsfield, S.B.; Allison, D.B.; Vasselli, J.R.; Pietrobelli, A.; Greenfield, D.; Nunez, C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: A randomized controlled trial. Jama 1998, 280, 1596–1600. [Google Scholar] [CrossRef]
- Parra-Vargas, M.; Sandoval-Rodriguez, A.; Rodriguez-Echevarria, R.; Dominguez-Rosales, J.; Santos-Garcia, A.; Armendariz-Borunda, J. Delphinidin ameliorates hepatic triglyceride accumulation in human HepG2 cells, but not in diet-induced obese mice. Nutrients 2018, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophy. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-Y.; Jung, U.J.; Park, T.; Yun, J.W.; Choi, M.-S. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes 2015, 64, 1658–1669. [Google Scholar] [CrossRef]
- Cho, A.-S.; Jeon, S.-M.; Kim, M.-J.; Yeo, J.; Seo, K.-I.; Choi, M.-S.; Lee, M.-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Volti, G.L.; Galvano, F. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, DB_101461. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Nishii, S.; Zaima, N.; Moriyama, T.; Kawamura, Y. Ellagic acid improves hepatic steatosis and serum lipid composition through reduction of serum resistin levels and transcriptional activation of hepatic ppara in obese, diabetic KK-Ay mice. Biochem. Biophy. Res. Commun. 2013, 434, 486–491. [Google Scholar] [CrossRef]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2013, 52, 559–568. [Google Scholar] [CrossRef]
- Kang, S.-W.; Kang, S.-I.; Shin, H.-S.; Yoon, S.-A.; Kim, J.-H.; Ko, H.-C.; Kim, S.-J. Sasa quelpaertensis Nakai extract and its constituent p-coumaric acid inhibit adipogenesis in 3T3-L1 cells through activation of the AMPK pathway. Food Chem. Toxicol. 2013, 59, 380–385. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Influence of oryzanol and ferulic acid on the lipid metabolism and antioxidative status in high fat-fed mice. J. Clin. Biochem. Nutr. 2010, 46, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharm. Phy. 2016, 43, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-C.; Ou, T.-T.; Wu, C.-H.; Wang, C.-J. Prevention of diet-induced hyperlipidemia and obesity by caffeic acid in C57BL/6 mice through regulation of hepatic lipogenesis gene expression. J. Agric. Food Chem. 2013, 61, 11082–11088. [Google Scholar] [CrossRef] [PubMed]
- Juman, S.; Yasui, N.; Okuda, H.; Ueda, A.; Negishi, H.; Miki, T.; Ikeda, K. Caffeic acid phenethyl ester suppresses the production of adipocytokines, leptin, tumor necrosis factor-alpha and resistin, during differentiation to adipocytes in 3T3-L1 cells. Biol. Pharm. Bull. 2011, 34, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Morales-Luna, E.; Perez-Ramirez, I.F.; Salgado, L.M.; Castano-Tostado, E.; Gomez-Aldapa, C.A.; Reynoso-Camacho, R. The main beneficial effect of roselle (Hibiscus sabdariffa) on obesity is not only related to its anthocyanin content. J. Sci. Food Agric. 2018, 99, 596–605. [Google Scholar] [CrossRef]
- Herranz-Lopez, M.; Fernandez-Arroyo, S.; Perez-Sanchez, A.; Barrajon-Catalan, E.; Beltran-Debon, R.; Menendez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.; Ferreira, I.C. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef]
- Villalpando-Arteaga, E.V.; Mendieta-Condado, E.; Esquivel-Solis, H.; Canales-Aguirre, A.A.; Galvez-Gastelum, F.J.; Mateos-Diaz, J.C.; Rodriguez-Gonzalez, J.A.; Marquez-Aguirre, A.L. Hibiscus sabdariffa L. aqueous extract attenuates hepatic steatosis through down-regulation of PPAR-gamma and SREBP-1c in diet-induced obese mice. Food Funct. 2013, 4, 618–626. [Google Scholar] [CrossRef]
- Peng, C.H.; Chyau, C.C.; Chan, K.C.; Chan, T.H.; Wang, C.J.; Huang, C.N. Hibiscus sabdariffa polyphenolic extract inhibits hyperglycemia, hyperlipidemia, and glycation-oxidative stress while improving insulin resistance. J. Agric. Food Chem. 2011, 59, 9901–9909. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Huang, C.-N.; Wang, C.-J.; Lee, Y.-J.; Chen, M.-L.; Peng, C.-H. Polyphenols of Hibiscus sabdariffa improved diabetic nephropathy via regulating the pathogenic markers and kidney functions of type 2 diabetic rats. J. Funct. Food 2013, 5, 810–819. [Google Scholar] [CrossRef]
- Si, L.Y.-N.; Ali, S.A.M.; Latip, J.; Fauzi, N.M.; Budin, S.B.; Zainalabidin, S. Roselle is cardioprotective in diet-induced obesity rat model with myocardial infarction. Life Sci. 2017, 191, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.G.; Chivandi, E.; Mojiminiyi, F.B.O.; Erlwanger, K.H. The response of male and female rats to a high-fructose diet during adolescence following early administration of Hibiscus sabdariffa aqueous calyx extracts. J. Dev. Orig. Hlth. Dis. 2017, 8, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Peng, C.H.; Yeh, D.M.; Kao, E.S.; Wang, C.J. Hibiscus sabdariffa extract inhibits obesity and fat accumulation, and improves liver steatosis in humans. Food Funct. 2014, 5, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Gurrola-Diaz, C.M.; Garcia-Lopez, P.M.; Sanchez-Enriquez, S.; Troyo-Sanroman, R.; Andrade-Gonzalez, I.; Gomez-Leyva, J.F. Effects of Hibiscus sabdariffa extract powder and preventive treatment (diet) on the lipid profiles of patients with metabolic syndrome (MeSy). Phytomedicine 2010, 17, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.K.; Kim, H.J.; Moon, S.R.; Shin, B.C.; Park, K.W.; Yang, H.O.; Kim, S.M.; Park, R. Hibiscus extract inhibits the lipid droplet accumulation and adipogenic transcription factors expression of 3T3-L1 preadipocytes. J. Altern. Complem. Med. 2003, 9, 499–504. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Xiao, P.; Yang, Z.; Sun, J.; Tian, J.; Chang, Z.; Li, X.; Zhang, B.; Ye, Y.; Ji, H.; Yu, E.; Xie, J. Silymarin inhibits adipogenesis in the adipocytes in grass carp Ctenopharyngodon idellus in vitro and in vivo. Fish Physiol. Biochem. 2017, 43, 1487–1500. [Google Scholar] [CrossRef]
- Akindahunsi, A.A.; Olaleye, M.T. Toxicological investigation of aqueous-methanolic extract of the calyces of Hibiscus sabdariffa L. J. Ethnopharmacol. 2003, 89, 161–164. [Google Scholar] [CrossRef]
- Hansawasdi, C.; Kawabata, J.; Kasai, T. α-Amylase inhibitors from roselle (Hibiscus sabdariffa Linn.) tea. Biosci. Biotech. Biochem. 2000, 64, 1041–1043. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Bagchi, D.; Stohs, S. Inhibition by natural dietary substances of gastrointestinal absorption of starch and sucrose in rats and pigs: 1. Acute studies. Int. J. Med. Sci. 2007, 4, 196. [Google Scholar] [CrossRef] [PubMed]
- Ofei, F. Obesity-a preventable disease. Ghana Med. J. 2005, 39, 98–101. [Google Scholar]
- Crosignani, A.; Zuin, M.; Allocca, M.; Del Puppo, M. Oxysterols in bile acid metabolism. Clin. Chim. Acta 2011, 412, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Chen, C.C.; Wang, W.H.; Hsu, J.D.; Yang, M.Y.; Wang, C.J. The protective effects of Hibiscus sabdariffa extract on CCl4-induced liver fibrosis in rats. Food Chem. Toxicol. 2006, 44, 336–343. [Google Scholar] [CrossRef]
- Hursel, R.; Westerterp-Plantenga, M.S. Thermogenic ingredients and body weight regulation. Int. J. Obes. 2010, 34, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.N.; Wu, T.Y.; Chau, C.F. Natural Dietary and Herbal Products in Anti-Obesity Treatment. Molecules 2016, 21, 1351. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Loizzo, M.R.; Nicoletti, M.; Menichini, F.; Conforti, F. Inhibition of key enzymes linked to obesity by preparations from Mediterranean dietary plants: Effects on α-amylase and pancreatic lipase activities. Plant Food Hum. Nutr. 2013, 68, 340–346. [Google Scholar] [CrossRef]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy 2000, 20, 270–279. [Google Scholar] [CrossRef]
- Farmer, S.R. Regulation of PPARgamma activity during adipogenesis. Int. J. Obes. 2005, 29, S13–S16. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Enikolopov, G.N.; Mintz, A.; Delbono, O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cell Dev. 2013, 22, 2298–2314. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Zhang, X.N.; Wang, W.; Xing, D.M.; Xie, W.D.; Su, H.; Du, L.J. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obes. 2007, 31, 1023–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D. The transcriptional basis of adipocyte development. Prostag. Leukotr. Ess. 2005, 73, 31–34. [Google Scholar] [CrossRef]
- Murase, Y.; Kobayashi, J.; Nohara, A.; Asano, A.; Yamaaki, N.; Suzuki, K.; Sato, H.; Mabuchi, H. Raloxifene promotes adipocyte differentiation of 3T3-L1 cells. Eur. J. Pharm. 2006, 538, 1–4. [Google Scholar] [CrossRef]
- Wagatsuma, A. Upregulation of gene encoding adipogenic transcriptional factors C/EBPα and PPARγ2 in denervated muscle. Exp. Physiol. 2006, 91, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosmann, G.; Barlette, A.G.; Dhamer, T.; Arcari, D.P.; Santos, J.C.; de Camargo, E.R.; Acedo, S.; Gambero, A.; Gnoatto, S.C.; Ribeiro, M.L. Phenolic compounds from mate (Ilex paraguariensis) inhibit adipogenesis in 3T3-L1 preadipocytes. Plant Food Hum. Nutr. 2012, 67, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Yang, H.; Seo, S.G.; Shin, S.H.; Chung, M.Y.; Kim, J.; Lee, S.J.; Lee, H.J.; Lee, K.W. Cocoa polyphenols suppress adipogenesis in vitro and obesity in vivo by targeting insulin receptor. Int. J. Obes. 2013, 37, 584–592. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.G.; Kang, Y.J.; Kwon, T.K.; Nam, J.O. Kahweol inhibits adipogenesis of 3T3-L1 adipocytes through downregulation of PPARgamma. Nat. Prod. Res. 2018, 32, 1216–1219. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, J.S.; Min, K.; Kwon, T.K.; Nam, J.O. Hispidulin inhibits adipogenesis in 3T3-L1 adipocytes through PPARgamma pathway. Chem. Biol. Interact. 2018, 293, 89–93. [Google Scholar] [CrossRef]
- Onyenekwe, P.; Ajani, E.; Ameh, D.; Gamaniel, K. Antihypertensive effect of roselle (Hibiscus sabdariffa) calyx infusion in spontaneously hypertensive rats and a comparison of its toxicity with that in Wistar rats. Cell Biochem. Funct. 1999, 17, 199–206. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Husaini, D.C.; Afonne, O.J. Testicular effects of sub-chronic administration of Hibiscus sabdariffa calyx aqueous extract in rats. Reprod. Toxicol. 2004, 18, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Ndu, O.O.; Nworu, C.S.; Ehiemere, C.O.; Ndukwe, N.C.; Ochiogu, I.S. Herb–drug interaction between the extract of Hibiscus sabdariffa L. and hydrochlorothiazide in experimental animals. J. Med. Food 2011, 14, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, J.; Maduenyi, A. Effect of zobo drink (Hibiscus sabdariffa water extract) on the pharmacokinetics of acetaminophen in human volunteers. Eur. J. Drug Metab. Pharmacok. 2004, 29, 25–29. [Google Scholar] [CrossRef]
- Khaghani, S.; Razi, F.; Yajloo, M.M.; Paknejad, M.; Shariftabrizi, A.; Pasalar, P. Selective cytotoxicity and apoptogenic activity of Hibiscus sabdariffa aqueous extract against MCF-7 human breast cancer cell line. J. Cancer Ther. 2011, 2, 394. [Google Scholar] [CrossRef]
- Fakeye, T.O.; Pal, A.; Bawankule, D.; Yadav, N.; Khanuja, S. Toxic effects of oral administration of extracts of dried calyx of Hibiscus sabdariffa Linn.(Malvaceae). Phytother. Res. 2009, 23, 412–416. [Google Scholar] [CrossRef] [PubMed]


| Bioactives | Chemical Formula | Studies on Obesity |
|---|---|---|
| Organic acids | ||
| Hydroxycitric acid | C6H8O8 | [41,42] |
| Hibiscus acid | C6H6O7 | - |
| Dimethyl hibiscus acid | C8H10O7 | - |
| Anthocyanins | ||
| Delphinidin-3-sambubioside (hibiscin) | C26H30O16 | - |
| Cyanidin-3-sambubioside (gossypicyanin) | C26H30O15 | - |
| Cyanidin-3,5-diglucoside | C26H30O15 | - |
| Delphinidin (anthocyanidin) | C15H11O7 | [43] |
| Flavonoids | ||
| Hibiscitrin (hibiscetin-3-glucoside) | C21H20O14 | - |
| Gossypitrin | C21H20O13 | - |
| Quercetin | C15H10O7 | [44,45] |
| Luteolin | C15H10O6 | [46] |
| Phenolic acid | ||
| Chlorogenic acid | C16H18O9 | [47,48] |
| Protocatechuic acid | C7H6O4 | [49] |
| Ellagic acid | C14H6O8 | [50,51] |
| P-Coumaric acid | C9H8O3 | [52] |
| Ferulic acid | C10H10O4 | [53,54] |
| Caffeic acid | C9H8O | [55,56] |
| Subject | Extraction Solvent | Effective Dose | Species | Observed Effect(s) | References |
|---|---|---|---|---|---|
| Mouse | Aqueous | 33.64 mg/kg | Swiss Webste (CFW) | Suppressed body weight gain in Ob/MSG mice by 9.6% and reduces glycaemia | [9] |
| Aqueous | 33 mg/kg | C57BL/6NHsd | Reduced fat tissue accumulation, normalized the glycemic index as well as reduced dyslipidemia | [61] | |
| Rat | Methanol | 100 mg/kg, 200 mg/kg | Sprague-Dawley rats | Significantly reduced the plasma level of triacylglycerol cholesterol, and LDL/HDL risk ratio | [62] |
| Methanol | 200 mg/kg | Sprague-Dawley rats | HPE inhibited fat deposition, hyperglycemia, serum advanced glycation end-products (AGE) | [63] | |
| Aqueous and Methanol | 3% of 750 mg (22.5mg) | Wistar Rats | Prevented increase in body weight and decreased adipocytes hyperplasia on rats fed a hypercaloric diet | [57] | |
| Ethanol | 5%, 10%, 15% (v/v) | Sprague-Dawley rats | Prevented increase in body weight, triglyceride, LDL and total lipids were lowered | [12] | |
| Aqueous | 100 mg/kg | Sprague-Dawley rats | Attenuated body weight gain, plasma leptin, cholesterol, triglycerol, LDL and systolic blood pressure | [64] | |
| Aqueous | 500 mg/kg | Sprague-Dawley rats | Prevented the hypercholesterolemia induced by dietary fructose | [65] | |
| Human | Aqueous | 1 g HSE capsule-dose | In vivo (human trial) | Decreased serum FFA level of the HSE group and improved liver steatosis | [66] |
| - | 100mg (Capsule) | In vivo (clinical study) | Patients with metabolic syndrome showed a significant reduction in total cholesterol and glucose level, with increased HDL level | [67] | |
| Cultured cells | Methanol | 0.25 or 0.5 mg/mL | 3T3-L1 pre-adipocytes cell | Suppressed differentiation and increased number of apoptotic cells in mature adipocytes | [8] |
| Aqueous | 2 mg/mL | 3T3-L1 pre-adipocytes cell | Inhibition of lipid accumulation by reducing intracellular lipid droplet during adipogenesis | [10] | |
| Aqueous | 100 mg/mL | 3T3-L1 pre-adipocytes cell | Reduced the expression of major adipogenic transcription factors including PPARγ and C/EBPα that regulate adipogenesis | [68] | |
| Aqueous and ethanol | 500 µg/mL aqueous extract and ethanol extract 10 µg/mL | 3T3-L1 pre-adipocytes | Inhibited lipid accumulation and adipogenic differentiation of pre-adipocytes | [58] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojulari, O.V.; Lee, S.G.; Nam, J.-O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. on Obesity. Molecules 2019, 24, 210. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010210
Ojulari OV, Lee SG, Nam J-O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. on Obesity. Molecules. 2019; 24(1):210. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010210
Chicago/Turabian StyleOjulari, Oyindamola Vivian, Seul Gi Lee, and Ju-Ock Nam. 2019. "Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. on Obesity" Molecules 24, no. 1: 210. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010210