Less Exploited GPCRs in Precision Medicine: Targets for Molecular Imaging and Theranostics
Abstract
:1. Introduction
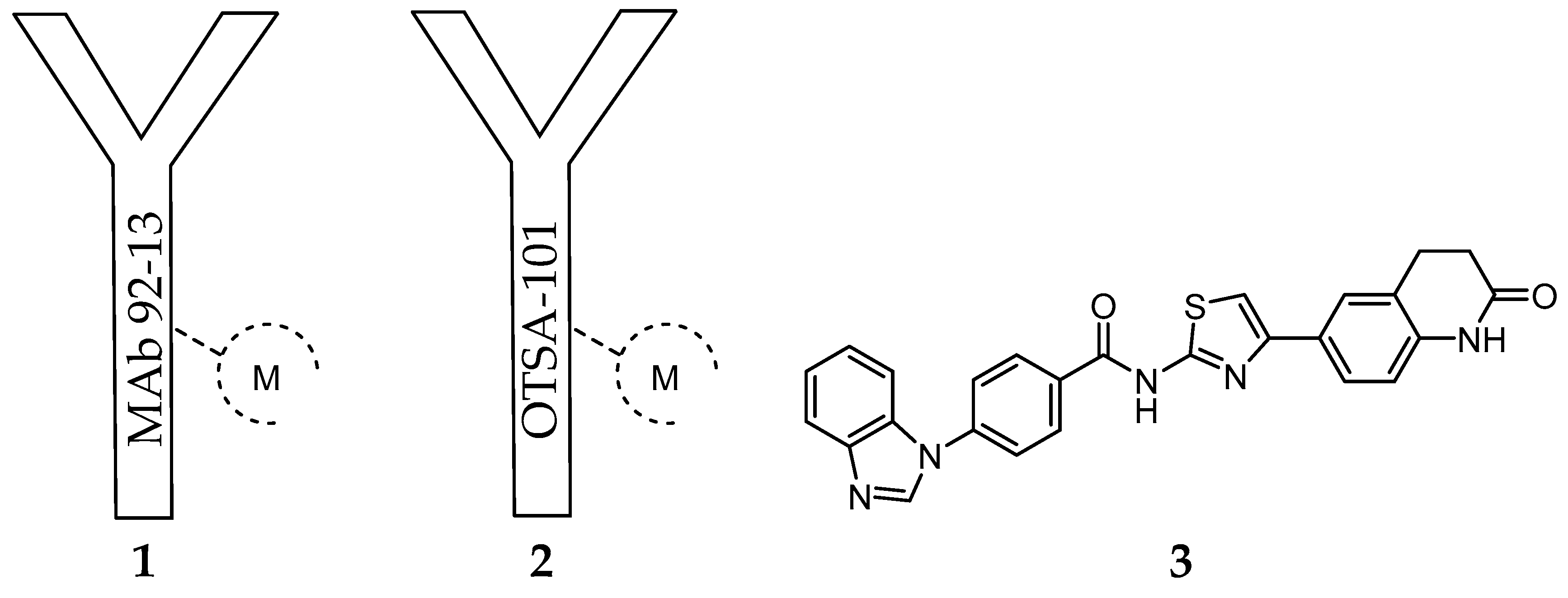
2. Frizzled Receptor (FZD)
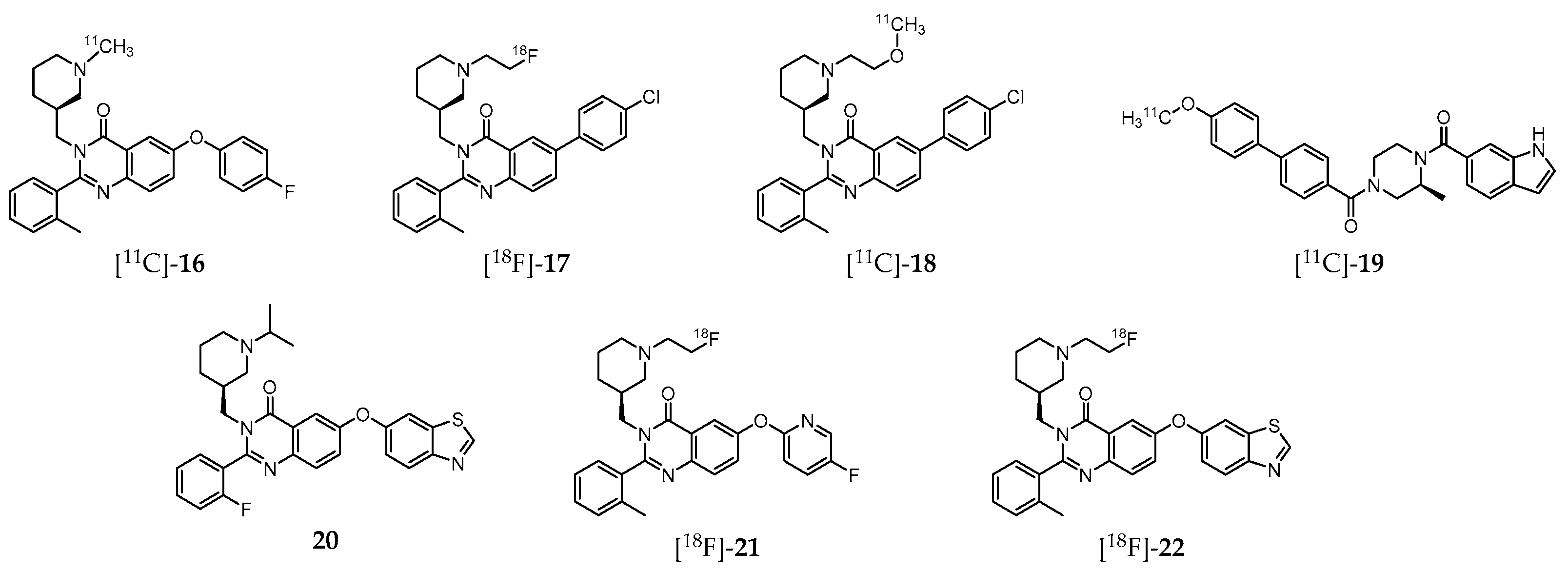
3. Ghrelin Receptor (GHSR-1a)
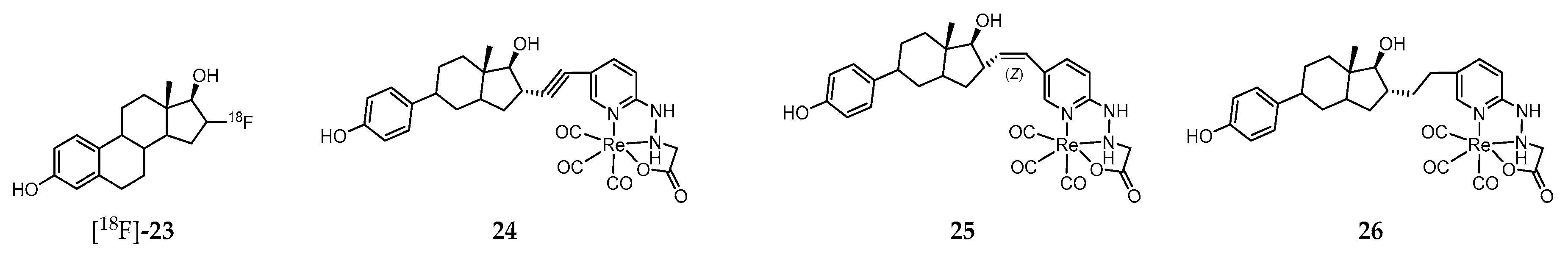
4. G protein-Coupled Estrogen Receptor (GPER)
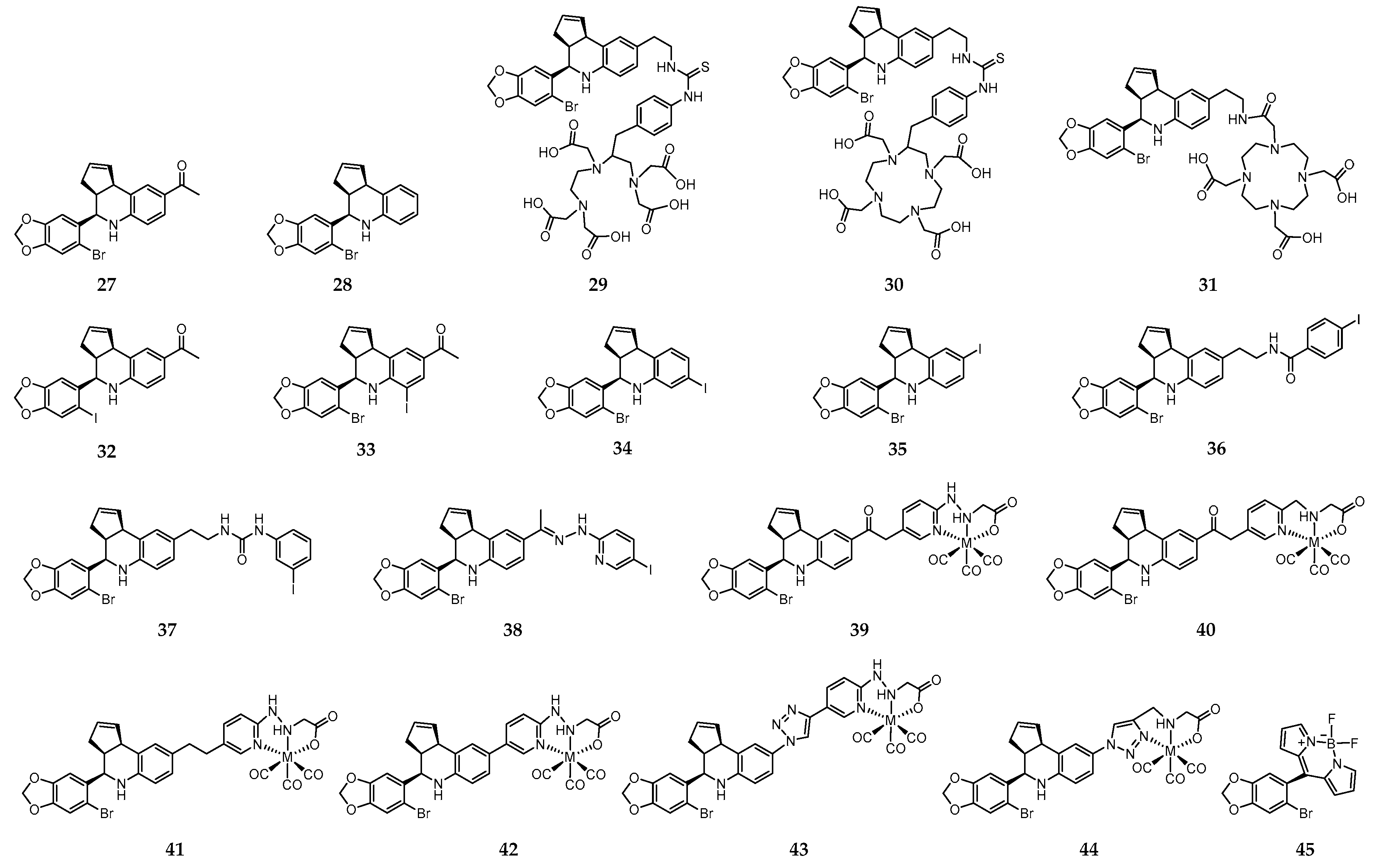
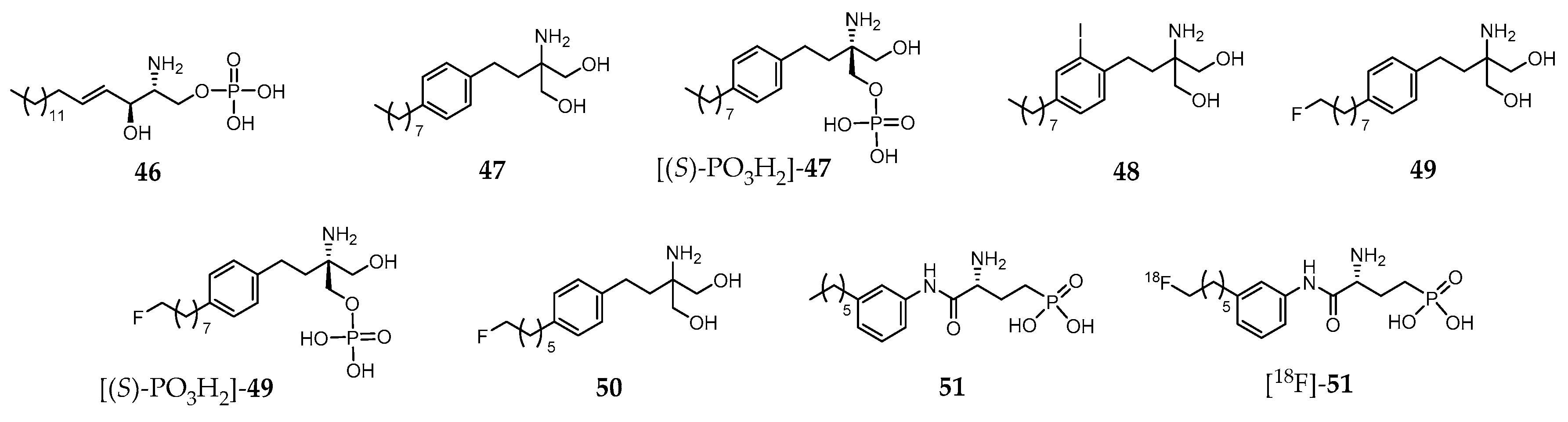
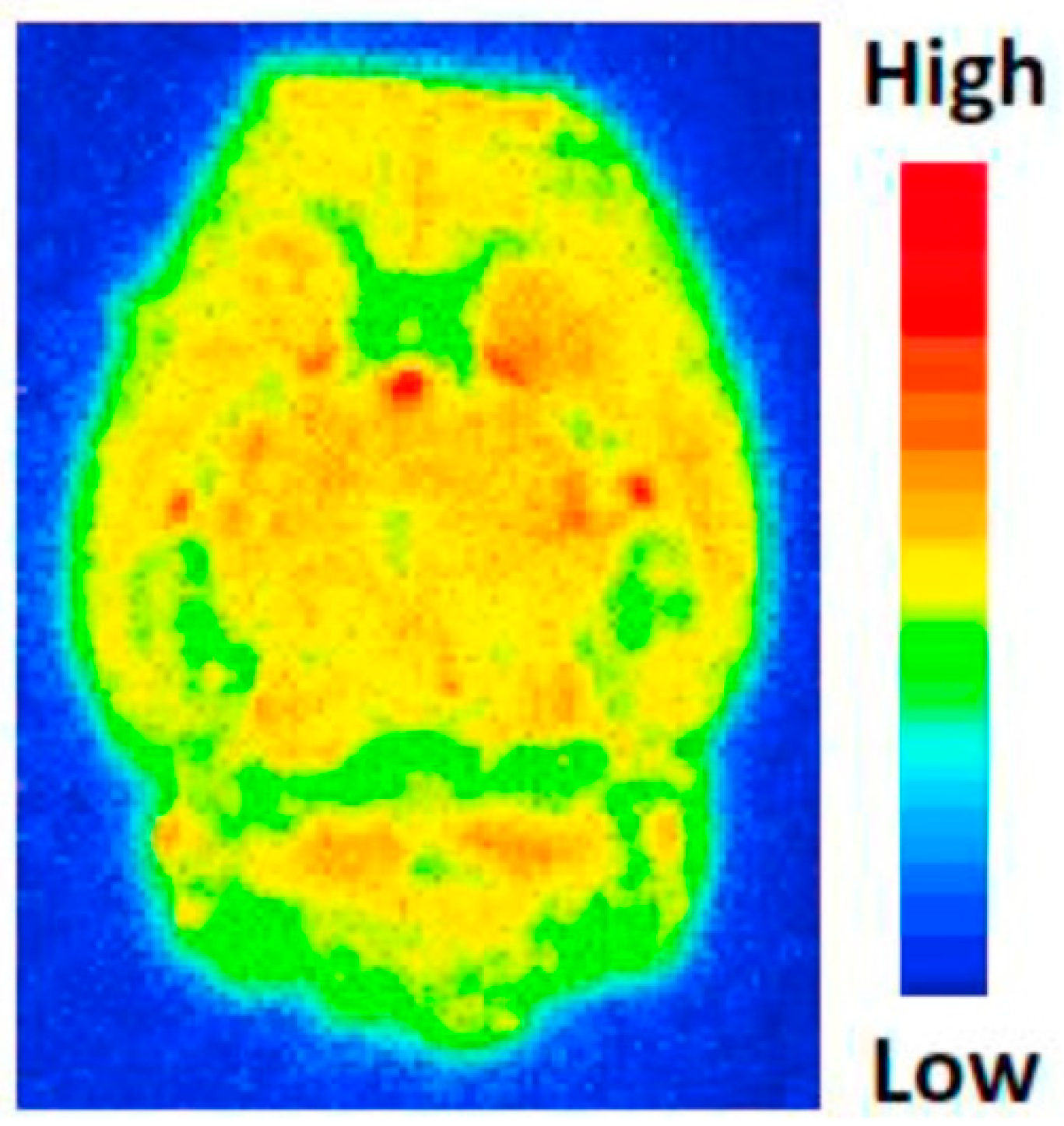
5. Sphingosine-1-Phosphate Receptor 1 (S1PR)
6. Concluding Remarks and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Collins, D.C.; Sundar, R.; Lim, J.S.J.; Yap, T.A. Towards Precision Medicine in the Clinic: From Biomarker Discovery to Novel Therapeutics. Trends Pharmacol. Sci. 2017, 38, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; de Bono, J. Targeted therapeutics for cancer treatment: Major progress towards personalised molecular medicine. Curr. Opin. Pharmacol. 2008, 8, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Moscow, J.A.; Fojo, T.; Schilsky, R.L. The Evidence Framework for Precision Cancer Medicine. Nat. Rev. Clin. Oncol. 2018, 15, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L.; Longo, D.L. Precision Medicine—Personalized, Problematic, and Promising. N. Engl. J. Med. 2015, 372, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Dugger, S.A.; Platt, A.; Goldstein, D.B. Drug development in the era of precision medicine. Nat. Rev. Drug Discov. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H. Targeted Radionuclide Therapy: An Evolution Toward Precision Cancer Treatment. Am. J. Roentgenol. 2017, 209, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Giardino, A.; Gupta, S.; Olson, E.; Sepulveda, K.; Lenchik, L.; Ivanidze, J.; Rakow-Penner, R.; Patel, M.J.; Subramaniam, R.M.; Ganeshan, D. Role of Imaging in the Era of Precision Medicine. Acad. Radiol. 2017, 24, 639–649. [Google Scholar] [CrossRef]
- Herold, C.J.; Lewin, J.S.; Wibmer, A.G.; Thrall, J.H.; Krestin, G.P.; Dixon, A.K.; Schoenberg, S.O.; Geckle, R.J.; Muellner, A.; Hricak, H. Imaging in the Age of Precision Medicine: Summary of the Proceedings of the 10th Biannual Symposium of the International Society for Strategic Studies in Radiology. Radiology 2016, 279, 226–238. [Google Scholar] [CrossRef]
- Ghasemi, M.; Nabipour, I.; Omrani, A.; Alipour, Z.; Assadi, M. Precision medicine and molecular imaging: New targeted approaches toward cancer therapeutic and diagnosis. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 310–327. [Google Scholar]
- McDermott, S.; Kilcoyne, A. Molecular imaging-its current role in cancer. QJM Int. J. Med. 2016, 109, 295–299. [Google Scholar] [CrossRef]
- Lu, Z.R.; Minko, T. Molecular imaging for precision medicine. Adv. Drug Deliv. Rev. 2017, 113, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ametamey, S.M.; Honer, M.; Schubiger, P.A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Wester, H.J. Nuclear imaging probes: From bench to bedside. Clin. Cancer Res. 2007, 13, 3470–3481. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. PET Radiopharmaceuticals for Personalized Medicine. Curr. Drug Targets 2016, 17, 1894–1907. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.T.; Chan, K.W.Y. Developing MR Probes for Molecular Imaging. Emerg. Appl. Mol. Imaging Oncol. 2014, 124, 297–327. [Google Scholar] [CrossRef]
- Sim, N.; Parker, D. Critical design issues in the targeted molecular imaging of cell surface receptors. Chem. Soc. Rev. 2015, 44, 2122–2134. [Google Scholar] [CrossRef] [Green Version]
- Herschman, H.R. Molecular imaging: Looking at problems, seeing solutions. Science 2003, 302, 605–608. [Google Scholar] [CrossRef]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Price, E.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Yordanova, A.; Eppard, E.; Krupig, S.; Bundschuh, R.A.; Schonberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. TheranosticsTheranostics in nuclear medicine practice. Oncotargets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef]
- Choudhury, P.; Gupta, M. Personalized & Precision Medicine in Cancer: A Theranostic Approach. Curr. Radiopharm. 2017, 10, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Wester, H.-J. Re-thinking the role of radiometal isotopes: Towards a future concept for theranostic radiopharmaceuticals. J. Label. Compd. Radiopharm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for Combined Imaging and Therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, J.R. Theranostic radiopharmaceuticals: Established agents in current use. Br. J. Radiol. 2018, 91. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Essler, M. It is time to move forward into the era of Theranostics. Ejnmmi Res. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Santos-Cuevas, C.; Ferro-Flores, G.; Garcia-Perez, F.O.; Jimenez-Mancilla, N.; Ramirez-Nava, G.; Ocampo-Garcia, B.; Luna-Gutierrez, M.; Azorin-Vega, E.; Davanzo, J.; Soldevilla-Gallardo, I. Lu-177-DOTA-HYNIC-Lys(Nal)-Urea-Glu: Biokinetics, Dosimetry, and Evaluation in Patients with Advanced Prostate Cancer. Contrast Media Mol. Imaging 2018. [Google Scholar] [CrossRef]
- Eberlein, U.; Cremonesi, M.; Lassmann, M. Individualized Dosimetry for Theranostics: Necessary, Nice to Have, or Counterproductive? J. Nucl. Med. 2017, 58, 97S–103S. [Google Scholar] [CrossRef]
- Li, T.T.; Ao, E.C.I.; Lambert, B.; Brans, B.; Vandenberghe, S.; Mok, G.S.P. Quantitative Imaging for Targeted Radionuclide Therapy Dosimetry—Technical Review. Theranostics 2017, 7, 4551–4565. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schioth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Hicks, R.J. Citius, Altius, Fortius: An Olympian Dream for Theranostics. J. Nucl. Med. 2017, 58, 194–195. [Google Scholar] [CrossRef]
- Graham, M.M.; Gu, X.M.; Ginader, T.; Breheny, P.; Sunderland, J.J. Ga-68-DOTATOC Imaging of Neuroendocrine Tumors: A Systematic Review and Metaanalysis. J. Nucl. Med. 2017, 58, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Ambrosini, V.; Herrmann, K.; Modlin, I. Current Concepts in Ga-68-DOTATATE Imaging of Neuroendocrine Neoplasms: Interpretation, Biodistribution, Dosimetry, and Molecular Strategies. J. Nucl. Med. 2017, 58, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Morigi, J.J.; Nanni, C.; Castellucci, P.; Fanti, S. Current status of PET imaging of neuroendocrine tumours (18F FDOPA, 68Ga tracers, 11C/18F-HTP). Q. J. Nucl. Med. Mol. Imaging 2015, 59, 58–69. [Google Scholar] [PubMed]
- Ambrosini, V.; Campana, D.; Tomassetti, P.; Fanti, S. Ga-68-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Moncayo, R.; Bombardieri, E.; Chiti, A. Somatostatin receptor SPECT. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, P.; Chandramahanti, S.; Kroiss, A.; Yu, R.; Ruszniewski, P.; Kumar, R.; Taieb, D. Nuclear imaging of neuroendocrine tumors with unknown primary: Why, when and how? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Cuccurullo, V.; Prisco, M.R.; Di Stasio, G.D.; Mansi, L. Nuclear Medicine in Patients with NET: Radiolabeled Somatostatin Analogues and their Brothers. Curr. Radiopharm. 2017, 10, 74–84. [Google Scholar] [CrossRef]
- Hope, T.A.; Pampaloni, M.H.; Flavell, R.R.; Nakakura, E.K.; Bergsland, E.K. Somatostatin receptor PET/MRI for the evaluation of neuroendocrine tumors. Clin. Transl. Imaging 2017, 5, 63–69. [Google Scholar] [CrossRef]
- Barrio, M.; Czernin, J.; Fanti, S.; Ambrosini, V.; Binse, I.; Du, L.; Eiber, M.; Herrmann, K.; Fendler, W.P. The Impact of Somatostatin Receptor-Directed PET/CT on the Management of Patients with Neuroendocrine Tumor: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2017, 58, 756–761. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J. Radionuclide Therapy for Neuroendocrine Tumors. Curr. Oncol. Rep. 2017, 19. [Google Scholar] [CrossRef]
- Pool, S.E.; Krenning, E.P.; Koning, G.A.; van Eijck, C.H.J.; Teunissen, J.J.M.; Kam, B.; Valkema, R.; Kwekkeboom, D.J.; de Jong, M. Preclinical and Clinical Studies of Peptide Receptor Radionuclide Therapy. Semin. Nucl. Med. 2010, 40, 209–218. [Google Scholar] [CrossRef]
- van Essen, M.; Sundin, A.; Krenning, E.P.; Kwekkeboom, D.J. Neuroendocrine tumours: The role of imaging for diagnosis and therapy. Nat. Rev. Endocrinol. 2014, 10, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Bluemel, C.; Allen-Auerbach, M.S.; Higuchi, T.; Herrmann, K. (68)Gallium- and (90)Yttrium-/(177)Lutetium: “theranostic twins” for diagnosis and treatment of NETs. Ann. Nucl. Med. 2015, 29, 1–7. [Google Scholar] [CrossRef]
- Fendler, W.P.; Baum, R.P. NTR Is the New SSTR? Perspective for Neurotensin Receptor 1 (NTR)-Directed Theranostics. J. Nucl. Med. 2017, 58, 934–935. [Google Scholar] [CrossRef] [PubMed]
- Emrarian, I.; Sadeghzadeh, N.; Abedi, S.M.; Abediankenari, S. New neurotensin analogue radiolabeled by 99m-technetium as a potential agent for tumor identification. Chem. Biol. Drug Des. 2018, 91, 304–313. [Google Scholar] [CrossRef]
- Maschauer, S.; Prante, O. Radiopharmaceuticals for imaging and endoradiotherapy of neurotensin receptor-positive tumors. J. Label. Compd. Radiopharm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Singh, A.; Schuchardt, C.; Kulkarni, H.R.; Klette, I.; Wiessalla, S.; Osterkamp, F.; Reineke, U.; Smerling, C. 177Lu-3BP-227 for neurotensin receptor 1-targeted therapy of metastatic pancreatic adenocarcinoma—First clinical results. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017. [Google Scholar] [CrossRef]
- Deng, H.; Wang, H.; Zhang, H.; Wang, M.; Giglio, B.; Ma, X.; Jiang, G.; Yuan, H.; Wu, Z.; Li, Z. Imaging Neurotensin Receptor in Prostate Cancer with Cu-64-Labeled Neurotensin Analogs. Mol. Imaging 2017, 16. [Google Scholar] [CrossRef]
- Schulz, J.; Rohracker, M.; Stiebler, M.; Goldschmidt, J.; Stober, F.; Noriega, M.; Pethe, A.; Lukas, M.; Osterkamp, F.; Reineke, U.; et al. Proof of Therapeutic Efficacy of a Lu-177-Labeled Neurotensin Receptor 1 Antagonist in a Colon Carcinoma Xenograft Model. J. Nucl. Med. 2017, 58, 936–941. [Google Scholar] [CrossRef]
- Schulz, J.; Rohracker, M.; Stiebler, M.; Goldschmidt, J.; Grosser, O.S.; Osterkamp, F.; Pethe, A.; Reineke, U.; Smerling, C.; Amthauer, H. Comparative Evaluation of the Biodistribution Profiles of a Series of Nonpeptidic Neurotensin Receptor-1 Antagonists Reveals a Promising Candidate for Theranostic Applications. J. Nucl. Med. 2016, 57, 1120–1123. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.D.; Fuscaldi, L.L.; Townsend, D.M.; Rubello, D.; de Barros, A.L.B. Radiolabeled bombesin derivatives for preclinical oncological imaging. Biomed. Pharmacother. 2017, 87, 58–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansi, R.; Minamimoto, R.; Macke, H.; Iagaru, A.H. Bombesin-Targeted PET of Prostate Cancer. J. Nucl. Med. 2016, 57, 67S–72S. [Google Scholar] [CrossRef] [PubMed]
- Mansi, R.; Fleischmann, A.; Macke, H.R.; Reubi, J.C. Targeting GRPR in urological cancers -from basic research to clinical application. Nat. Rev. Urol. 2013, 10, 235–244. [Google Scholar] [CrossRef]
- Sancho, V.; Di Florio, A.; Moody, T.W.; Jensen, R.T. Bombesin Receptor-Mediated Imaging and Cytotoxicity: Review and Current Status. Curr. Drug Deliv. 2011, 8, 79–134. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.P.J.; van Weerden, W.M.; Bangma, C.; Krenning, E.P.; de Jong, M. Peptide receptor imaging of prostate cancer with radiolabelled bombesin analogues. Methods 2009, 48, 200–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, N.; Paquette, M.; Ait-Mohand, S.; Dumulon-Perreault, V.; Guerin, B. Evaluation of a novel GRPR antagonist for prostate cancer PET imaging: Cu-64-DOTHA(2)-PEG-RM26. Nucl. Med. Biol. 2018, 56, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.; Castellucci, P.; Cerci, J.J.; Fanti, S. New aspects of molecular imaging in prostate cancer. Methods 2017, 130, 36–41. [Google Scholar] [CrossRef]
- Wieser, G.; Popp, I.; Rischke, H.C.; Drendel, V.; Grosu, A.L.; Bartholoma, M.; Weber, W.A.; Mansi, R.; Wetterauer, U.; Schultze-Seemann, W.; et al. Diagnosis of recurrent prostate cancer with PET/CT imaging using the gastrin-releasing peptide receptor antagonist Ga-68-RM2: Preliminary results in patients with negative or inconclusive F-18 Fluoroethylcholine-PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1463–1472. [Google Scholar] [CrossRef]
- Sonni, I.; BarattO, L.; Iagaru, A. Imaging of Prostate Cancer Using Gallium-68-Labeled Bombesin. PET Clin. 2017, 12, 159–171. [Google Scholar] [CrossRef]
- Maffioli, L.; Florimonte, L.; Costa, D.C.; Castanheira, J.C.; Grana, C.; Luster, M.; Bodei, L.; Chinol, M. New radiopharmaceutical agents for the treatment of castration-resistant prostate cancer. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 420–438. [Google Scholar]
- Moreno, P.; Ramos-Alvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maina, T.; Nock, B.A.; Kulkarni, H.; Singh, A.; Baum, R.P. Theranostic Prospects of Gastrin-Releasing Peptide Receptor-Radioantagonists in Oncology. PET Clin. 2017, 12, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Zhang, H.W.; La Rosa, S.; Jebiwott, S.; Desai, P.; Kimm, S.; Scherz, A.; O’Donoghue, J.A.; Weber, W.A.; Coleman, J.A. Bombesin Antagonist-Based Radiotherapy of Prostate Cancer Combined with WST-11 Vascular Targeted Photodynamic Therapy. Clin. Cancer Res. 2017, 23, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Nock, B.A. From Bench to Bed New Gastrin-Releasing Peptide Receptor-Directed Radioligands and Their Use in Prostate Cancer. PET Clin. 2017, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.S.; Bandari, R.P.; Jiang, Z.R.; Smith, C.J. Lutetium-177 Labeled Bombesin Peptides for Radionuclide Therapy. Curr. Radiopharm. 2016, 9, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Roosenburg, S.; Laverman, P.; van Delft, F.L.; Boerman, O.C. Radiolabeled CCK/gastrin peptides for imaging and therapy of CCK2 receptor-expressing tumors. Amino Acids 2011, 41, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Konijnenberg, M.W.; KolencPeitl, P.; Garnuszek, P.; Nock, B.A.; Kaloudi, A.; Kroselj, M.; Zaletel, K.; Maecke, H.; Mansi, R.; et al. Preclinical pharmacokinetics, biodistribution, radiation dosimetry and toxicity studies required for regulatory approval of a phase I clinical trial with In-111-CP04 in medullary thyroid carcinoma patients. Eur. J. Pharm. Sci. 2016, 91, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Trejtnar, F.; Laznickova, A.; Laznicek, M.; Novy, Z.; Maina, T.; Nock, B.A.; Behe, M. Distribution, Elimination, and Renal Handling of (99m)Technetium-Demogastrin 1. Cancer Biother. Radiopharm. 2012, 27, 169–174. [Google Scholar] [CrossRef]
- Roy, J.; Putt, K.S.; Coppola, D.; Leon, M.E.; Khalil, F.K.; Centeno, B.A.; Clark, N.; Stark, V.E.; Morse, D.L.; Low, P.S. Assessment of cholecystokinin 2 receptor (CCK2R) in neoplastic tissue. Oncotarget 2016, 7, 14605–14615. [Google Scholar] [CrossRef] [Green Version]
- Pawlak, D.; Rangger, C.; Peitl, P.K.; Garnuszek, P.; Maurin, M.; Ihli, L.; Kroselj, M.; Maina, T.; Maecke, H.; Erba, P.; et al. From preclinical development to clinical application: Kit formulation for radiolabelling the minigastrin analogue CP04 with In-111 for a first-in-human clinical trial. Eur. J. Pharm. Sci. 2016, 85, 1–9. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Valkema, R.; Krenning, E.P.; de Jong, M.; Maina, T. Impact of clinically tested NEP/ACE inhibitors on tumor uptake of (111)ln-DOTA MG11-first estimates for clinical translation. Ejnmmi Res. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Krenning, E.P.; de Jong, M.; Maina, T. Improving the In Vivo Profile of Minigastrin Radiotracers: A Comparative Study Involving the Neutral Endopeptidase Inhibitor Phosphoramidon. Cancer Biother. Radiopharm. 2016, 31, 20–28. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Krenning, E.P.; de Jong, M.; Maina, T. Tc-99m-labeled gastrins of varying peptide chain length: Distinct impact of NEP/ACE-inhibition on stability and tumor uptake in mice. Nucl. Med. Biol. 2016, 43, 347–354. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Krenning, E.P.; Maina, T.; De Jong, M. Radiolabeled gastrin/CCK analogs in tumor diagnosis: Towards higher stability and improved tumor targeting. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 287–302. [Google Scholar] [PubMed]
- Nock, B.A.; Maina, T.; Krenning, E.P.; de Jong, M. “To Serve and Protect”: Enzyme Inhibitors as Radiopeptide Escorts Promote Tumor Targeting. J. Nucl. Med. 2014, 55, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Maschauer, S.; Brennauer, A.; Tripal, P.; Koglin, N.; Dittrich, R.; Bernhardt, G.; Kuwert, T.; Wester, H.J.; Buschauer, A.; et al. Prototypic F-18-Labeled Argininamide-Type Neuropeptide Y Y1R Antagonists as Tracers for PET Imaging of Mammary Carcinoma. ACS Med. Chem. Lett. 2017, 8, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pan, J.; Lin, K.S.; Dude, I.; Lau, J.; Zeisler, J.; Merkens, H.; Jenni, S.; Guerin, B.; Benard, F. Targeting the Neuropeptide Y1 Receptor for Cancer Imaging by Positron Emission Tomography Using Novel Truncated Peptides. Mol. Pharm. 2016, 13, 3657–3664. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Maschauer, S.; Kuwert, T.; Beck-Sickinger, A.G.; Prante, O. Synthesis and in Vitro and in Vivo Evaluation of an F-18-Labeled Neuropeptide Y Analogue for Imaging of Breast Cancer by PET. Mol. Pharm. 2015, 12, 1121–1130. [Google Scholar] [CrossRef]
- Morgat, C.; Mishra, A.K.; Varshney, R.; Allard, M.; Fernandez, P.; Hindie, E. Targeting Neuropeptide Receptors for Cancer Imaging and Therapy: Perspectives with Bombesin, Neurotensin, and Neuropeptide-Y Receptors. J. Nucl. Med. 2014, 55, 1650–1657. [Google Scholar] [CrossRef] [Green Version]
- Winterdahl, M.; Audrain, H.; Landau, A.M.; Smith, D.F.; Bonaventure, P.; Shoblock, J.R.; Carruthers, N.; Swanson, D.; Bender, D. PET Brain Imaging of Neuropeptide Y2 Receptors Using N-C-11-Methyl-JNJ-31020028 in Pigs. J. Nucl. Med. 2014, 55, 635–639. [Google Scholar] [CrossRef]
- Hostetler, E.D.; Sanabria-Bohorquez, S.; Fan, H.; Zeng, Z.Z.; Gantert, L.; Williams, M.; Miller, P.; O’Malley, S.; Kameda, M.; Ando, M.; et al. Synthesis, characterization, and monkey positron emission tomography (PET) studies of F-18 Y1-973, a PET tracer for the neuropeptide Y Y1 receptor. Neuroimage 2011, 54, 2635–2642. [Google Scholar] [CrossRef] [PubMed]
- Chatenet, D.; Cescato, R.; Waser, B.; Erchegyi, J.; Rivier, J.E.; Reubi, J.C. Novel dimeric DOTA-coupled peptidic Y-1-receptor antagonists for targeting of neuropeptide Y receptor-expressing cancers. Ejnmmi Res. 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Guerin, B.; Dumulon-Perreault, V.; Tremblay, M.C.; Ait-Mohand, S.; Fournier, P.; Dubuc, C.; Authier, S.; Benard, F. Lys(DOTA)(4) BVD15, a novel and potent neuropeptide Y analog designed for Y-1 receptor-targeted breast tumor imaging. Bioorg. Med. Chem. Lett. 2010, 20, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Zwanziger, D.; Bohme, I.; Javed, M.; Naseer, H.; Hyder, S.W.; Beck-Sickinger, A.G. Breast-Cancer Diagnosis by Neuropeptide Y Analogues: From Synthesis to Clinical Application. Angew. Chem.-Int. Ed. 2010, 49, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Winterdahl, M.; Audrain, H.; Landau, A.; Bonaventure, P.; Shoblock, J.; Carruthers, N.; Swanson, D.; Bender, D. PET brain imaging of neuropeptide Y2 receptors. Eur. Neuropsychopharmacol. 2013, 23, S263–S264. [Google Scholar] [CrossRef]
- Higuchi, T.; Rischpler, C.; Fukushima, K.; Isoda, T.; Xia, J.S.; Javadi, M.S.; Szabo, Z.; Dannals, R.F.; Mathews, W.B.; Bengel, F.M. Targeting of Endothelin Receptors in the Healthy and Infarcted Rat Heart Using the PET Tracer F-18-FBzBMS. J. Nucl. Med. 2013, 54, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Werner, R.A.; Javadi, M.S.; Maya, Y.; Decker, M.; Lapa, C.; Herrmann, K.; Higuchi, T. Radionuclide Imaging of Neurohormonal System of the Heart. Theranostics 2015, 5, 545–558. [Google Scholar] [CrossRef] [Green Version]
- Michel, K.; Buther, K.; Law, M.P.; Wagner, S.; Schober, O.; Hermann, S.; Schafers, M.; Riemann, B.; Holtke, C.; Kopka, K. Development and Evaluation of Endothelin-A Receptor (Radio)Ligands for Positron Emission Tomography. J. Med. Chem. 2011, 54, 939–948. [Google Scholar] [CrossRef]
- Bai, M.; Bornhop, D.J. Recent Advances in Receptor-Targeted Fluorescent Probes for In Vivo Cancer Imaging. Curr. Med. Chem. 2012, 19, 4742–4758. [Google Scholar] [CrossRef] [PubMed]
- Holtke, C.; Faust, A.; Breyholz, H.J.; Kopka, K.; Schober, O.; Riemann, B.; Bremer, C.; Schafers, M.; Wagner, S. Non-Invasive Approaches to Visualize the Endothelin Axis In Vivo Using State-of-the-Art Molecular Imaging Modalities. Mini-Rev. Med. Chem. 2009, 9, 1580–1595. [Google Scholar] [CrossRef]
- Holtke, C.; Law, M.P.; Wagner, S.; Kopka, K.; Faust, A.; Breyholz, H.J.; Schober, O.; Bremer, C.; Riemann, B.; Schafers, M. PET-compatible endothelin receptor radioligands: Synthesis and first in vitro and in vivo studies. Bioorg. Med. Chem. 2009, 17, 7197–7208. [Google Scholar] [CrossRef] [PubMed]
- Mathews, W.B.; Murugesan, N.; Xia, J.; Scheffell, U.; Hilton, J.; Ravert, H.T.; Dannals, R.F.; Szabo, Z. Synthesis and in vivo evaluation of novel PET radioligands for imaging the endothelin-A receptor. J. Nucl. Med. 2008, 49, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Lin, K.S.; Benard, F. Molecular Imaging and Radionuclide Therapy of Melanoma Targeting the Melanocortin 1 Receptor. Mol. Imaging 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Zhang, Z.X.; Lin, K.S.; Pan, J.H.; Dude, I.; Hundal-Jabal, N.; Colpo, N.; Benard, F. Preclinical Melanoma Imaging with Ga-68-Labeled alpha-Melanocyte-Stimulating Hormone Derivatives Using PET. Theranostics 2017, 7, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.; Fernandez, M.; Oddone, N.; Zhang, X.L.; Gallazzi, F.; Cerecetto, H.; Gambini, J.P.; Porcal, W.; Cabral, P.; Quinn, T.P. The Effect of A Hexanoic Acid Linker Insertion on the Pharmacokinetics and Tumor Targeting Properties of the Melanoma Imaging Agent 99mTc-HYNIC-cycMSH. Anti-Cancer Agents Med. Chem. 2017, 17, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Oliveira, B.L.; Correia, J.D.G.; Oliveira, M.C.; Jimenez, M.A.; Santos, I.; Raposinho, P.D. Influence of the Bifunctional Chelator on the Pharmacokinetic Properties of Tc-99m(CO)3-Labeled Cyclic alpha-Melanocyte Stimulating Hormone Analog. J. Med. Chem. 2013, 56, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Raposinho, P.D.; Correia, J.D.G.; Oliveira, M.C.; Santos, I. Melanocortin-1 Receptor-Targeting With Radiolabeled Cyclic alpha-Melanocyte-Stimulating Hormone Analogs for Melanoma Imaging. Biopolymers 2010, 94, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Pan, Y.; Cheng, Z. Molecular Probes for Malignant Melanoma Imaging. Curr. Pharm. Biotechnol. 2010, 11, 590–602. [Google Scholar] [CrossRef]
- Quinn, T.; Zhang, X.; Miao, Y. Targeted melanoma imaging and therapy with radiolabeled alpha-melanocyte stimulating hormone peptide analogues. G. Ital. Dermatol. Venereol. 2010, 145, 245–258. [Google Scholar]
- Nagy, G.; Denes, N.; Kis, A.; Szabo, J.P.; Berenyi, E.; Garai, I.; Bai, P.; Hajdu, I.; Szikra, D.; Trencsenyi, G. Preclinical evaluation of melanocortin-1 receptor (MC1-R) specific Ga-68-and Sc-44-labeled DOTA-NAPamide in melanoma imaging. Eur. J. Pharm. Sci. 2017, 106, 336–344. [Google Scholar] [CrossRef]
- Carta, D.; Salvarese, N.; Morellato, N.; Gao, F.; Sihver, W.; Pietzsch, H.J.; Biondi, B.; Ruzza, P.; Refosco, F.; Carpanese, D.; et al. Melanoma targeting with Tc-99m(N)(PNP3)-labeled alpha-melanocyte stimulating hormone peptide analogs: Effects of cyclization on the radiopharmaceutical properties. Nucl. Med. Biol. 2016, 43, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Sihver, W.; Jurischka, C.; Bergmann, R.; Haase-Kohn, C.; Mosch, B.; Steinbach, J.; Carta, D.; Bolzati, C.; Calderan, A.; et al. Radiopharmacological characterization of Cu-64-labeled alpha-MSH analogs for potential use in imaging of malignant melanoma. Amino Acids 2016, 48, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Rangger, C.; Helbok, A.; Ocak, M.; Radolf, T.; Andreae, F.; Virgolini, I.J.; Von Guggenberg, E.; Decristoforo, C. Design and Evaluation of Novel Radiolabelled VIP Derivatives for Tumour Targeting. Anticancer Res. 2013, 33, 1537–1546. [Google Scholar] [PubMed]
- Tang, B.; Yong, X.; Xie, R.; Li, Q.W.; Yang, S.M. Vasoactive intestinal peptide receptor- based imaging and treatment of tumors (Review). Int. J. Oncol. 2014, 44, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Kumar, P.; Trabulsi, E.J.; Kim, S.; McCue, P.A.; Intenzo, C.; Berger, A.; Gomella, L.; Thakur, M.L. VPAC1 Targeted Cu-64-TP3805 kit preparation and its evaluation. Nucl. Med. Biol. 2017, 51, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Trabulsi, E.J.; Gomella, L.; Kim, S.; McCue, P.; Intenzo, C.; Birbe, R.; Gandhe, A.; Kumar, P.; Thakur, M. VPAC1 Targeted Cu-64-TP3805 Positron Emission Tomography Imaging of Prostate Cancer: Preliminary Evaluation in Man. Urology 2016, 88, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Funke, U.; Vugts, D.J.; Janssen, B.; Spaans, A.; Kruijer, P.S.; Lammertsma, A.A.; Perk, L.R.; Windhorst, A.D. 11C-labeled and 18F-labeled PET ligands for subtype-specific imaging of histamine receptors in the brain. J. Label. Compd. Radiopharm. 2013, 56, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Schou, M.; Varnas, K.; Jureus, A.; Ahlgren, C.; Malmquist, J.; Haggkvist, J.; Tari, L.; Wesolowski, S.S.; Throner, S.R.; Brown, D.G.; et al. Discovery and Preclinical Validation of C-11 AZ13153556, a Novel Probe for the Histamine Type 3 Receptor. ACS Chem. Neurosci. 2016, 7, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.Y.; Champion, S.; Wyper, D.; Dewar, D.; Pimlott, S. Assessment of I-125 WYE-230949 as a Novel Histamine H-3 Receptor Radiopharmaceutical. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Maeda, J.; Tokunaga, M.; Hanyu, M.; Kawamura, K.; Ohmichi, M.; Nakamura, T.; Nagai, Y.; Seki, C.; Kimura, Y.; et al. Development of TASP0410457 (TASP457), a novel dihydroquinolinone derivative as a PET radioligand for central histamine H-3 receptors. Ejnmmi Res. 2016, 6. [Google Scholar] [CrossRef]
- Kimura, Y.; Seki, C.; Ikoma, Y.; Ichise, M.; Kawamura, K.; Takahata, K.; Moriguchi, S.; Nagashima, T.; Ishii, T.; Kitamura, S.; et al. C-11 TASP457, a novel PET ligand for histamine H-3 receptors in human brain. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, M.; Kawamura, K.; Takei, M.; Furutsuka, K.; Shiomi, S.; Fujishiro, T.; Ogawa, M.; Nengaki, N.; Hashimoto, H.; Fukumura, T.; et al. Radiosynthesis and quality control of C-11 TASP457 as a clinically useful PET ligand for imaging of histamine H-3 receptors in human brain. Nucl. Med. Biol. 2016, 43, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Nebel, N.; Maschauer, S.; Kuwert, T.; Hocke, C.; Prante, O. In Vitro and In Vivo Characterization of Selected Fluorine-18 Labeled Radioligands for PET Imaging of the Dopamine D3 Receptor. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Prante, O.; Maschauer, S.; Banerjee, A. Radioligands for the dopamine receptor subtypes. J. Label. Compd. Radiopharm. 2013, 56, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Mach, R.H. Small Molecule Receptor Ligands for PET Studies of the Central Nervous System-Focus on G Protein Coupled Receptors. Semin. Nucl. Med. 2017, 47, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Prante, O. Subtype-Selective Dopamine Receptor Radioligands for PET Imaging: Current Status and Recent Developments. Curr. Med. Chem. 2012, 19, 3957–3966. [Google Scholar] [CrossRef] [PubMed]
- Saulin, A.; Savli, M.; Lanzenberger, R. Serotonin and molecular neuroimaging in humans using PET. Amino Acids 2012, 42, 2039–2057. [Google Scholar] [CrossRef] [PubMed]
- Paterson, L.M.; Kornum, B.R.; Nutt, D.J.; Pike, V.W.; Knudsen, G.M. 5-HT radioligands for human brain imaging with PET and SPECT. Med. Res. Rev. 2013, 33, 54–111. [Google Scholar] [CrossRef] [PubMed]
- Hazari, P.P.; Pandey, A.; Chaturvedi, S.; Mishra, A.K. New Trends and Current Status of Positron-Emission Tomography and Single-Photon-Emission Computerized Tomography Radioligands for Neuronal Serotonin Receptors and Serotonin Transporter. Bioconj. Chem. 2017, 28, 2647–2672. [Google Scholar] [CrossRef]
- Tyacke, R.J.; Nutt, D.J. Optimising PET approaches to measuring 5-HT release in human brain. Synapse 2015, 69, 505–511. [Google Scholar] [CrossRef]
- Hargreaves, R.J.; Rabiner, E.A. Translational PET imaging research. Neurobiol. Dis. 2014, 61, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Honer, M.; Gobbi, L.; Martarello, L.; Comley, R.A. Radiologand development for molecular imaging of the central nervous system with positron emission tomography. Drug Discov. Today 2014, 19, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Gunn, R.N.; Rabiner, E.A. Imaging in Central Nervous System Drug Discovery. Semin. Nucl. Med. 2017, 47, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Declercq, L.D.; Vandenberghe, R.; Van Laere, K.; Verbruggen, A.; Bormans, G. Drug Development in Alzheimer’s Disease: The Contribution of PET and SPECT. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Buiter, H.J.C.; Windhorst, A.D.; Huisman, M.C.; Yaqub, M.; Knol, D.L.; Fisher, A.; Lammertsma, A.A.; Leysen, J.E. C-11 AF150(S), an agonist PET ligand for M1 muscarinic acetylcholine receptors. Ejnmmi Res. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Ravasi, L.; Tokugawa, J.; Nakayama, T.; Seidel, J.; Sokoloff, L.; Eckelman, W.C.; Kiesewetter, D.O. Imaging of the muscarinic acetylcholine neuroreceptor in rats with the M2 selective agonist F-18 FP-TZTP. Nucl. Med. Biol. 2012, 39, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Moustapha, M.E.; Motaleb, M.A.; Ibrahim, I.T. Synthesis of Tc-99m-oxybutynin for M-3-receptor-mediated imaging of urinary bladder. J. Radioanal. Nucl. Chem. 2011, 287, 35–40. [Google Scholar] [CrossRef]
- van Oosten, E.M.; Wilson, A.A.; Mamo, D.C.; Pollock, B.G.; Mulsant, B.H.; Houle, S.; Vasdev, N. Towards the development of new subtype-specific muscarinic receptor radiopharmaceuticals—Radiosynthesis and ex vivo biodistribution of F-18 3-(4-(2-(2-(2-fluoroethoxy)ethoxy)ethylthio)-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,6-tetrahydropyridine. Can. J. Chem.-Revue Can. Chim. 2010, 88, 1222–1232. [Google Scholar] [CrossRef]
- Renard, P.Y.; Jean, L. Probing the cholinergic system to understand neurodegenerative diseases. Futur. Med. Chem. 2017, 9, 131–133. [Google Scholar] [CrossRef]
- Radeke, H.S.; Purohit, A.; Harris, T.D.; Hanson, K.; Jones, R.; Hu, C.; Yalamanchili, P.; Hayes, M.; Yu, M.; Guaraldi, M.; et al. Synthesis and Cardiac Imaging of F-18-Ligands Selective for beta(1)-Adrenoreceptors. ACS Med. Chem. Lett. 2011, 2, 650–655. [Google Scholar] [CrossRef]
- Kopka, K.; Law, M.P.; Breyholz, H.J.; Faust, A.; Holtke, C.; Riemann, B.; Schober, O.; Schafers, M.; Wagner, S. Non-invasive molecular imaging of beta-adrenoceptors in vivo: Perspectives for PET-radioligands. Curr. Med. Chem. 2005, 12, 2057–2074. [Google Scholar] [CrossRef] [PubMed]
- Wollenweber, T.; Bengel, F.M. Cardiac Molecular Imaging. Semin. Nucl. Med. 2014, 44, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Lehto, J.; Virta, J.R.; Oikonen, V.; Roivainen, A.; Luoto, P.; Arponen, E.; Helin, S.; Hietamaki, J.; Holopainen, A.; Kailajarvi, M.; et al. Test-retest reliability of C-11-ORM-13070 in PET imaging of alpha(2C)-adrenoceptors in vivo in the human brain. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Luoto, P.; Suilamo, S.; Oikonen, V.; Arponen, E.; Helin, S.; Herttuainen, J.; Hietamaki, J.; Holopainen, A.; Kailajarvi, M.; Peltonen, J.M.; et al. C-11-ORM-13070, a novel PET ligand for brain alpha(2C)-adrenoceptors: Radiometabolism, plasma pharmacokinetics, whole-body distribution and radiation dosimetry in healthy men. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Arponen, E.; Helin, S.; Marjamaki, P.; Gronroos, T.; Holm, P.; Loyttyniemi, E.; Nagren, K.; Scheinin, M.; Haaparanta-Solin, M.; Sallinen, J.; et al. A PET Tracer for Brain alpha(2C) Adrenoceptors, C-11-ORM-13070: Radiosynthesis and Preclinical Evaluation in Rats and Knockout Mice. J. Nucl. Med. 2014, 55, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Evens, N.; Bormans, G.M. Non-Invasive Imaging of the Type 2 Cannabinoid Receptor, Focus on Positron Emission Tomography. Curr. Top. Med. Chem. 2010, 10, 1527–1543. [Google Scholar] [CrossRef] [PubMed]
- Horti, A.G.; Van Laere, K. Development of Radioligands for In Vivo Imaging of Type 1 Cannabinoid Receptors (CB1) in Human Brain. Curr. Pharm. Des. 2008, 14, 3363–3383. [Google Scholar] [CrossRef]
- Navarro, G.; Morales, P.; Rodriguez-Cueto, C.; Fernandez-Ruiz, J.; Jagerovic, N.; Franco, R. Targeting Cannabinoid CB2 Receptors in the Central Nervous System. Medicinal Chemistry Approaches with Focus on Neurodegenerative Disorders. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Moldovan, R.P.; Teodoro, R.; Gao, Y.J.; Deuther-Conrad, W.; Kranz, M.; Wang, Y.C.; Kuwabara, H.; Nakano, M.; Valentine, H.; Fischer, S.; et al. Development of a High-Affinity PET Radioligand for Imaging Cannabinoid Subtype 2 Receptor. J. Med. Chem. 2016, 59, 7840–7855. [Google Scholar] [CrossRef]
- Caille, F.; Cacheux, F.; Peyronneau, M.A.; Jego, B.; Jaumain, E.; Pottier, G.; Ullmer, C.; Grether, U.; Winkeler, A.; Dolle, F.; et al. From Structure-Activity Relationships on Thiazole Derivatives to the In Vivo Evaluation of a New Radiotracer for Cannabinoid Subtype 2 PET Imaging. Mol. Pharm. 2017, 14, 4064–4078. [Google Scholar] [CrossRef]
- Moldovan, R.P.; Hausmann, K.; Deuther-Conrad, W.; Brust, P. Development of Highly Affine and Selective Fluorinated Cannabinoid Type 2 Receptor Ligands. ACS Med. Chem. Lett. 2017, 8, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Ahamed, M.; van Veghel, D.; Ullmer, C.; Van Laere, K.; Verbruggen, A.; Bormans, G.M. Synthesis, Biodistribution and in vitro Evaluation of Brain Permeable High Affinity Type 2 Cannabinoid Receptor Agonists C-11 MA2 and F-18 MA3. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Vuorimaa, A.; Rissanen, E.; Airas, L. In Vivo PET Imaging of Adenosine 2A Receptors in Neuroinflammatory and Neurodegenerative Disease. Contrast Media Mol. Imaging 2017. [Google Scholar] [CrossRef]
- Mishina, M.; Ishiwata, K. Adenosine Receptor PET Imaging in Human Brain. Adenosine Recept. Neurol. Psychiatr. 2014, 119, 51–69. [Google Scholar] [CrossRef]
- van Waarde, A.; Dierckx, R.; Zhou, X.Y.; Khanapur, S.; Tsukada, H.; Ishiwata, K.; Luurtsema, G.; de Vries, E.F.J.; Elsinga, P.H. Potential Therapeutic Applications of Adenosine A(2A) Receptor Ligands and Opportunities for A(2A) Receptor Imaging. Med. Res. Rev. 2018, 38, 5–56. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Granziera, C.; Hooker, J.M.; Loggia, M.L. In Vivo Imaging of Human Neuroinflammation. ACS Chem. Neurosci. 2016, 7, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Masino, S.A.; Kawamura, M.; Ruskin, D.N. Adenosine Receptors and Epilepsy: Current Evidence and Future Potential. In Adenosine Receptors in Neurology and Psychiatry; Mori, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 119, pp. 233–255. [Google Scholar]
- Gruber, S.; Ametamey, S.M. Imaging the glutamate receptor subtypes-Much achieved, and still much to do. Drug Discov. Today Technol. 2017, 25, 27–36. [Google Scholar] [CrossRef]
- Majo, V.J.; Prabhakaran, J.; Mann, J.J.; Kumar, J.S.D. PET and SPECT tracers for glutamate receptors. Drug Discov. Today 2013, 18, 173–184. [Google Scholar] [CrossRef]
- Li, S.Y.; Huang, Y.Y. In Vivo Imaging of the Metabotropic Glutamate Receptor 1 (mGluR1) with Positron Emission Tomography: Recent Advance and Perspective. Curr. Med. Chem. 2014, 21, 113–123. [Google Scholar] [CrossRef]
- Kuil, J.; Buckle, T.; van Leeuwen, F.W.B. Imaging agents for the chemokine receptor 4 (CXCR4). Chem. Soc. Rev. 2012, 41, 5239–5261. [Google Scholar] [CrossRef]
- Bluemel, C.; Hahner, S.; Heinze, B.; Fassnacht, M.; Kroiss, M.; Bley, T.A.; Wester, H.J.; Kropf, S.; Lapa, C.; Schirbel, A.; et al. Investigating the Chemokine Receptor 4 as Potential Theranostic Target in Adrenocortical Cancer Patients. Clin. Nucl. Med. 2017, 42, E29–E34. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.K.; Stolzenburg, A.; Hanscheid, H.; Schirbel, A.; Luckerath, K.; Schottelius, M.; Wester, H.J.; Lapa, C. Chemokine receptor—Directed imaging and therapy. Methods 2017, 130, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Kircher, S.; Schirbel, A.; Rosenwald, A.; Kropf, S.; Pelzer, T.; Walles, T.; Buck, A.K.; Weber, W.A.; Wester, H.J.; et al. Targeting CXCR4 with 68Ga Pentixafor: A suitable theranostic approach in pleural mesothelioma. Oncotarget 2017, 8, 96732–96737. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbok, S.M.; Stenzel, J.; Otto, T.; Helldorff, H.V.; Bergner, C.; Kurth, J.; Polei, S.; Lindner, T.; Rauer, R.; Hohn, A.; et al. Ga-68 pentixafor for CXCR4 imaging in a PC-3 prostate cancer xenograft model—Comparison with F-18 FDG PET/CT, MRI and ex vivo receptor expression. Oncotarget 2017, 8, 95606–95619. [Google Scholar] [CrossRef] [PubMed]
- Walenkamp, A.M.E.; Lapa, C.; Herrmann, K.; Wester, H.J. CXCR4 Ligands: The Next Big Hit? J. Nucl. Med. 2017, 58, 77S–82S. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.; Singh, B.; Basher, R.; Singh, H.; Bal, A.; Kapoor, R.; Arora, S.K.; Wester, H.J.; Mittal, B.R.; Behera, D. 68Ga-Pentixafor PET/CT demonstrating higher CXCR4 density in small cell lung carcinoma than in non-small cell variant. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Weich, A.; Higuchi, T.; Schmid, J.S.; Schirbel, A.; Lassmann, M.; Wild, V.; Rudelius, M.; Kudlich, T.; Herrmann, K.; et al. Imaging of Chemokine Receptor 4 Expression in Neuroendocrine Tumors—A Triple Tracer Comparative Approach. Theranostics 2017, 7, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Habringer, S.; Herhaus, P.; Schottelius, M.; Lapa, C.; Istvanffy, R.; Gotze, K.; Steiger, K.; Vick, B.; Peschel, C.; Oostendorp, R.; et al. Peptide-Receptor Radiotherapy with CXCR4-Targeting Pentixather Reduces Leukemia Burden in Acute Leukemia PDX and Patients. Blood 2016, 128, 4055. [Google Scholar]
- Schulte, G. International Union of Basic and Clinical Pharmacology. LXXX. The Class Frizzled Receptors. Pharmacol. Rev. 2010, 62, 632–667. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.M.; Chen, Z.; Fu, L. Frizzled Receptors as Potential Therapeutic Targets in Human Cancers. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Schulte, G.; Wright, S.C. Frizzleds as GPCRs—More Conventional Than We Thought! Trends Pharmacol. Sci. 2018, 39, 828–842. [Google Scholar] [CrossRef]
- Nagayama, S.; Katagiri, T.; Tsunoda, T.; Hosaka, T.; Nakashima, Y.; Araki, N.; Kusuzaki, K.; Nakayama, T.; Tsuboyama, T.; Nakamura, T.; et al. Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res. 2002, 62, 5859–5866. [Google Scholar] [PubMed]
- Fukukawa, C.; Hanaoka, H.; Nagayama, S.; Tsunoda, T.; Toguchida, J.; Endo, K.; Nakamura, Y.; Katagiri, T. Radioimmunotherapy of human synovial sarcoma using a monoclonal antibody against FZD10. Cancer Sci. 2008, 99, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Hanaoka, H.; Katagiri, T.; Fukukawa, C.; Yoshioka, H.; Yamamoto, S.; Iida, Y.; Higuchi, T.; Oriuchi, N.; Paudyal, B.; Paudyal, P.; et al. Radioimmunotherapy of solid tumors targeting a cell-surface protein, FZD10: Therapeutic efficacy largely depends on radiosensitivity. Ann. Nucl. Med. 2009, 23, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Giraudet, A.-L.; Cassier, P.A.; Iwao-Fukukawa, C.; Garin, G.; Badel, J.-N.; Kryza, D.; Chabaud, S.; Gilles-Afchain, L.; Clapisson, G.; Desuzinges, C.; et al. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer 2018, 18, 646. [Google Scholar] [CrossRef] [PubMed]
- Li, H.K.; Sugyo, A.; Tsuji, A.B.; Morokoshi, Y.; Minegishi, K.; Nagatsu, K.; Kanda, H.; Harada, Y.; Nagayama, S.; Katagiri, T.; et al. alpha-particle therapy for synovial sarcoma in the mouse using an astatine-211-labeled antibody against frizzled homolog 10. Cancer Sci. 2018, 109, 2302–2309. [Google Scholar] [CrossRef]
- Lindegren, S.; Frost, S.; Baeck, T.; Haglund, E.; Elgvist, J.; Jensen, H. Direct procedure for the production of At-211-labeled antibodies with an epsilon-lysyl-3-(trimethylstannyl)benzamide immunoconjugate. J. Nucl. Med. 2008, 49, 1537–1545. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, W.Y.; Ananthan, S.; Suto, M.J.; Li, Y.H. Discovery of novel frizzled-7 inhibitors by targeting the receptor’s transmembrane domain. Oncotarget 2017, 8, 91459–91470. [Google Scholar] [CrossRef]
- Janda, C.Y.; Waghray, D.; Levin, A.M.; Thomas, C.; Garcia, K.C. Structural Basis of Wnt Recognition by Frizzled. Science 2012, 337, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Dann, C.E.; Hsieh, J.C.; Rattner, A.; Sharma, D.; Nathans, J.; Leahy, D.J. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 2001, 412, 86–90. [Google Scholar] [CrossRef]
- Lee, H.J.; Bao, J.; Miller, A.; Zhang, C.; Wu, J.B.; Baday, Y.C.; Guibao, C.; Li, L.; Wu, D.Q.; Zheng, J.J. Structure-based Discovery of Novel Small Molecule Wnt Signaling Inhibitors by Targeting the Cysteine-rich Domain of Frizzled. J. Biol. Chem. 2015, 290, 30596–30606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, A.P.; Bonner, T.I.; Foord, S.M.; Harmar, A.J.; Neubig, R.R.; Pin, J.P.; Spedding, M.; Kojima, M.; Kangawa, K. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol. Rev. 2005, 57, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Bodart, V.; Febbraio, M.; Demers, A.; McNicoll, N.; Pohankova, P.; Perreault, A.; Sejlitz, T.; Escher, E.; Silverstein, R.L.; Lamontagne, D.; et al. CD36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart. Circ. Res. 2002, 90, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.; Pons, N.; Ghe, C.; Catapano, F.; Granata, R.; Ghigo, E. Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type 1a growth hormone secretagogue receptor. Eur. J. Pharmacol. 2004, 498, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Delhanty, P.J.D.; van der Eerden, B.C.J.; van der Velde, M.; Gauna, C.; Pols, H.A.P.; Jahr, H.; Chiba, H.; van der Lely, A.J.; van Leeuwen, J. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J. Endocrinol. 2006, 188, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, N.M.; Gill, D.A.S.; Davies, R.; Loveridge, N.; Houston, P.A.; Robinson, I.; Wells, T. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 2004, 145, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.R.Y.; Smith, R.G. The growth hormone secretagogue receptor. In Vitamins and Hormones, Ghrelin; Litwack, G., Ed.; Elsevier Academic Press Inc.: San Diego, FL, USA, 2008; Volume 77, p. 47. [Google Scholar]
- Bednarek, M.A.; Feighner, S.D.; Pong, S.S.; McKee, K.K.; Hreniuk, D.L.; Silva, M.V.; Warren, V.A.; Howard, A.D.; Van der Ploeg, L.H.Y.; Heck, J.V. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: Minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J. Med. Chem. 2000, 43, 4370–4376. [Google Scholar] [CrossRef]
- Mani, B.K.; Zigman, J.M. Ghrelin as a Survival Hormone. Trends Endocrinol. Metab. 2017, 28, 843–854. [Google Scholar] [CrossRef]
- Lin, T.C.; Hsiao, M. Ghrelin and cancer progression. Biochim. Biophys. Acta-Rev. Cancer 2017, 1868, 51–57. [Google Scholar] [CrossRef]
- Beiras-Fernandez, A.; Kreth, S.; Weis, F.; Ledderose, C.; Pottinger, T.; Dieguez, C.; Beiras, A.; Reichart, B. Altered myocardial expression of ghrelin and its receptor (GHSR-1a) in patients with severe heart failure. Peptides 2010, 31, 2222–2228. [Google Scholar] [CrossRef]
- Katugampola, S.D.; Pallikaros, Z.; Davenport, A.P. I-125-His(9) -Ghrelin, a novel radioligand for localizing GHS orphan receptors in human and rat tissue; up-regulation of receptors with atherosclerosis. Br. J. Pharmacol. 2001, 134, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.; Papotti, M.; Locatelli, V.; Ghigo, E.; Deghenghi, R. Binding of I-125-labeled ghrelin to membranes from human hypothalamus and pituitary gland. J. Endocrinol. Investig. 2001, 24, RC7–RC9. [Google Scholar] [CrossRef]
- Harrold, J.A.; Dovey, T.; Cai, X.J.; Halford, J.C.G.; Pinkney, J. Autoradiographic analysis of ghrelin receptors in the rat hypothalamus. Brain Res. 2008, 1196, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Wojciuk, G.; Kruszewski, M. DTPA-(PABn)-Leu(5)-des-acyl ghrelin(1-5) as a new carrier of radionuclides and potential precursor of radiopharmaceuticals. Nucl. Med. Commun. 2018, 39, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kozminski, P.; Gniazdowska, E. Synthesis and in vitro/in vivo evaluation of novel mono- and trivalent technetium-99m labeled ghrelin peptide complexes as potential diagnostic radiopharmaceuticals. Nucl. Med. Biol. 2015, 42, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Chollet, C.; Bergmann, R.; Pietzsch, J.; Beck-Sickinger, A.G. Design, Evaluation, and Comparison of Ghrelin Receptor Agonists and Inverse Agonists as Suitable Radiotracers for PET Imaging. Bioconj. Chem. 2012, 23, 771–784. [Google Scholar] [CrossRef]
- Carlie, L.C.; Savita, D.; Leonard, G.L. Evaluation of Ga-68-DOTA ghrelin (1-19) in LNCaP prostate carcinoma. Nucl. Med. Biol. 2014, 41, 638. [Google Scholar] [CrossRef]
- Charron, C.L.; Hou, J.N.; McFarland, M.S.; Dhanvantari, S.; Kovacs, M.S.; Luyt, L.G. Structure Activity Study of Ghrelin(1-8) Resulting in High Affinity Fluorine-Bearing Ligands for the Ghrelin Receptor. J. Med. Chem. 2017, 60, 7256–7266. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Holst, B.; Frimurer, T.M.; Mokrosinski, J.; Halkjaer, T.; Cullberg, K.B.; Underwood, C.R.; Schwartz, T.W. Overlapping Binding Site for the Endogenous Agonist, Small-Molecule Agonists, and Ago-allosteric Modulators on the Ghrelin Receptor. Mol. Pharmacol. 2009, 75, 44–59. [Google Scholar] [CrossRef]
- McGirr, R.; McFarland, M.S.; McTavish, J.; Luyt, L.G.; Dhanvantari, S. Design and characterization of a fluorescent ghrelin analog for imaging the growth hormone secretagogue receptor 1a. Regul. Pept. 2011, 172, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; McFarland, M.S.; Nesbitt, R.L.; Williams, A.K.; Chan, S.; Gomez-Lemus, J.; Autran-Gomez, A.M.; Al-Zahrani, A.; Chin, J.L.; Izawa, J.I.; et al. Ghrelin receptor as a novel imaging target for prostatic neoplasms. Prostate 2012, 72, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.A.F.; McGirr, R.; Charlton, C.L.; Kagan, D.B.; Hoffman, L.M.; Luyt, L.G.; Dhanvantari, S. Characterization of a far-red analog of ghrelin for imaging GHS-R in P19-derived cardiomyocytes. Peptides 2014, 54, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosita, D.; Dewit, M.A.; Luyt, L.G. Fluorine and Rhenium Substituted Ghrelin Analogues as Potential Imaging Probes for the Growth Hormone Secretagogue Receptor. J. Med. Chem. 2009, 52, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.; Horti, A.G.; Ravert, H.T.; Holt, D.P.; Finley, P.; Scheffel, U.; Dannals, R.F.; Wahl, R.L. Synthesis and in vivo evaluation of (S)-6-(4-fluorophenoxy)-3-((1-C-11 methylpiperidin-3-yl)methyl)-2-o-tol ylquinazolin-4(3H)-one, a potential PET tracer for growth hormone secretagogue receptor (GHSR). Bioorg. Med. Chem. 2011, 19, 2368–2372. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Fujinaga, M.; Shimoda, Y.; Yamasaki, T.; Zhang, Y.D.; Hatori, A.; Xie, L.; Wakizaka, H.; Kumata, K.; Ohkubo, T.; et al. Developing new PET tracers to image the growth hormone secretagogue receptor 1a (GHS-R1a). Nucl. Med. Biol. 2017, 52, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.Q.; Kovacs, M.S.; Dhanvantari, S.; Luyt, L.G. Development of Candidates for Positron Emission Tomography (PET) Imaging of Ghrelin Receptor in Disease: Design, Synthesis, and Evaluation of Fluorine-Bearing Quinazolinone Derivatives. J. Med. Chem. 2018, 61, 1261–1275. [Google Scholar] [CrossRef]
- Barton, M.; Filardo, E.J.; Lolait, S.J.; Thomas, P.; Maggiolini, M.; Prossnitz, E.R. Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives. J. Steroid Biochem. Mol. Biol. 2018, 176, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Prossnitz, E.R.; Arterburn, J.B. International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacol. Rev. 2015, 67, 505–540. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Kiyono, Y.; Mori, T.; Okazawa, H. Positron Emission Tomography Radiopharmaceuticals for Sex Steroid Hormone Receptor Imaging. Curr. Med. Chem. 2012, 19, 3266–3270. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.N.; Gligorov, J.; Nataf, V.; Montravers, F.; Huchet, V.; Michaud, L.; Ohnona, J.; Balogova, S.; Cussenot, O.; Darai, E.; et al. Current applications of PET imaging of sex hormone receptors with a fluorinated analogue of estradiol or of testosterone. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 4–17. [Google Scholar] [PubMed]
- van Kruchten, M.; de Vries, E.G.E.; Brown, M.; de Vries, E.F.J.; Glaudemans, A.; Dierckx, R.; Schroder, C.P.; Hospers, G.A.P. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 2013, 14, E465–E475. [Google Scholar] [CrossRef]
- Ramesh, C.; Bryant, B.; Nayak, T.; Revankar, C.M.; Anderson, T.; Carlson, K.E.; Katzenellenbogen, J.A.; Sklar, L.A.; Norenberg, J.P.; Prossnitz, E.R.; et al. Linkage effects on binding affinity and activation of GPR30 and estrogen receptors ER alpha/beta with tridentate pyridin-2-yl hydrazine tricarbonyl-Re/Tc-99m(I) chelates. J. Am. Chem. Soc. 2006, 128, 14476–14477. [Google Scholar] [CrossRef]
- Nayak, T.K.; Hathaway, H.J.; Ramesh, C.; Arterburn, J.B.; Dai, D.; Sklar, L.A.; Norenberg, J.P.; Prossnitz, E.R. Preclinical development of a neutral, estrogen receptor-targeted, tridentate Tc-99m(I)-estradiol-pyridin-2-yl hydrazine derivative for imaging of breast and endometrial cancers. J. Nucl. Med. 2008, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Bologa, C.G.; Revankar, C.M.; Young, S.M.; Edwards, B.S.; Arterburn, J.B.; Kiselyov, A.S.; Parker, M.A.; Tkachenko, S.E.; Savchuck, N.P.; Sklar, L.A.; et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2006, 2, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.K.; Burai, R.; Ramesh, C.; Petrie, W.K.; Alcon, S.N.; Nayak, T.K.; Bologa, C.G.; Leitao, A.; Brailoiu, E.; Deliu, E.; et al. In vivo effects of a GPR30 antagonist. Nat. Chem. Biol. 2009, 5, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, T.K.; Dennis, M.K.; Ramesh, C.; Burai, R.; Atcher, R.W.; Sklar, L.A.; Norenberg, J.P.; Hathaway, H.J.; Arterburn, J.B.; Prossnitz, E.R. Influence of Charge on Cell Permeability and Tumor Imaging of GPR30-Targeted In-111-Labeled Nonsteroidal Imaging Agents. ACS Chem. Biol. 2010, 5, 681–690. [Google Scholar] [CrossRef]
- Ramesh, C.; Nayak, T.K.; Burai, R.; Dennis, M.K.; Hathaway, H.J.; Sklar, L.A.; Prossnitz, E.R.; Arterburn, J.B. Synthesis and Characterization of Iodinated Tetrahydroquinolines Targeting the G Protein-Coupled Estrogen Receptor GPR30. J. Med. Chem. 2010, 53, 1004–1014. [Google Scholar] [CrossRef]
- Burai, R.; Ramesh, C.; Nayak, T.K.; Dennis, M.K.; Bryant, B.K.; Prossnitz, E.R.; Arterburn, J.B. Synthesis and Characterization of Tricarbonyl-Re/Tc(I) Chelate Probes Targeting the G Protein-Coupled Estrogen Receptor GPER/GPR30. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Nayak, T.K.; Ramesh, C.; Hathaway, H.J.; Norenberg, J.P.; Arterburn, J.B.; Prossnitz, E.R. GPER-Targeted, Tc-99m-Labeled, Nonsteroidal Ligands Demonstrate Selective Tumor Imaging and In Vivo Estrogen Binding. Mol. Cancer Res. 2014, 12, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Papalia, T.; Lappano, R.; Barattucci, A.; Pisano, A.; Bruno, G.; Santolla, M.F.; Campagna, S.; De Marco, P.; Puntoriero, F.; De Francesco, E.M.; et al. A Bodipy as a luminescent probe for detection of the G protein estrogen receptor (GPER). Org. Biomol. Chem. 2015, 13, 10437–10441. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010, 9, 883–897. [Google Scholar] [CrossRef]
- Rosenberg, A.J.; Liu, H.; Jin, H.J.; Yue, X.Y.; Riley, S.; Brown, S.J.; Tu, Z.D. Design, Synthesis, and In Vitro and In Vivo Evaluation of an F-18-Labeled Sphingosine 1-Phosphate Receptor 1 (S1P(1)) PET Tracer. J. Med. Chem. 2016, 59, 6201–6220. [Google Scholar] [CrossRef] [PubMed]
- Briard, E.; Orain, D.; Beerli, C.; Billich, A.; Streiff, M.; Bigaud, M.; Auberson, Y.P. BZM055, an Iodinated Radiotracer Candidate for PET and SPECT Imaging of Myelin and FTY720 Brain Distribution. ChemMedChem 2011, 6, 667–677. [Google Scholar] [CrossRef]
- Shaikh, R.S.; Schilson, S.S.; Wagner, S.; Hermann, S.; Keul, P.; Levkau, B.; Schafers, M.; Haufe, G. Synthesis and Evaluation of Fluorinated Fingolimod (FTY720) Analogues for Sphingosine-1-Phosphate Receptor Molecular Imaging by Positron Emission Tomography. J. Med. Chem. 2015, 58, 3471–3484. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.P.; Wagner, S.; Keul, P.; Hermann, S.; Levkau, B.; Schafers, M.; Haufe, G. Synthesis of fluorinated analogues of sphingosine-1-phosphate antagonists as potential radiotracers for molecular imaging using positron emission tomography. Bioorg. Med. Chem. 2014, 22, 5168–5181. [Google Scholar] [CrossRef]
- Jin, H.J.; Yang, H.; Liu, H.; Zhang, Y.X.; Zhang, X.; Rosenberg, A.J.; Liu, Y.J.; Lapi, S.E.; Tu, Z.D. A promising carbon-11-labeled sphingosine-1-phosphate receptor 1-specific PET tracer for imaging vascular injury. J. Nucl. Cardiol. 2017, 24, 558–570. [Google Scholar] [CrossRef]
- Liu, H.; Jin, H.J.; Yue, X.Y.; Luo, Z.H.; Liu, C.L.; Rosenberg, A.J.; Tu, Z.D. PET Imaging Study of S1PR1 Expression in a Rat Model of Multiple Sclerosis. Mol. Imaging Biol. 2016, 18, 724–732. [Google Scholar] [CrossRef]
- Liu, H.; Jin, H.J.; Yue, X.Y.; Han, J.B.; Baum, P.; Abendschein, D.R.; Tu, Z.D. PET Study of Sphingosine-1-Phosphate Receptor 1 Expression in Response to Vascular Inflammation in a Rat Model of Carotid Injury. Mol. Imaging 2017, 16. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Jin, H.J.; Han, J.B.; Yue, X.Y.; Yang, H.; Zayed, M.A.; Gropler, R.J.; Tu, Z.D. Upregulated Sphingosine 1-Phosphate Receptor 1 Expression in Human and Murine Atherosclerotic Plaques. Mol. Imaging Biol. 2018, 20, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.H.; Rosenberg, A.J.; Liu, H.; Han, J.B.; Tu, Z.D. Syntheses and in vitro evaluation of new S1PR1 compounds and initial evaluation of a lead F-18 radiotracer in rodents. Eur. J. Med. Chem. 2018, 150, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Bar-Or, A.; Cree, B.A.C.; Fox, R.J.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnold, D.L.; Arnould, S.; Scherz, T.; et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 2018, 391, 1263–1273. [Google Scholar] [CrossRef]
- Briard, E.; Rudolph, B.; Desrayaud, S.; Krauser, J.A.; Auberson, Y.P. MS565: A SPECT Tracer for Evaluating the Brain Penetration of BAF312 (Siponimod). ChemMedChem 2015, 10, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.; Barret, O.; Alagille, D.; Morley, T.; Papin, C.; Maguire, R.P.; Briard, E.; Auberson, Y.P.; Tamagnan, G. Brain distribution of MS565, an imaging analogue of siponimod (BAF312), in non-human primates. J. Neurol. 2014, 261, S333. [Google Scholar]
- Bhattacharya, S.K.; Cameron, K.O.k.; Fernando, D.P.; Kung, D.W.-S.; Londregan, A.T.; Mcclure, K.F.; Simila, S.T.M. 2,3-Dihydro-1H-inden-1-yl-2,7-diazaspiro[3.6] Nonane and Their Use as Antagonists or Inverse Agonists of the Ghrelin Receptor. WO Application WO2011114271A8, 8 November 2012. [Google Scholar]
- Luyt, L.G.; Fowkes, M.M. Peptidomimetics for Imaging the Ghrelin Receptor. U.S. Patent 15/578,105, 7 June 2018. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco Machado, J.; Silva, R.D.; Melo, R.; G. Correia, J.D. Less Exploited GPCRs in Precision Medicine: Targets for Molecular Imaging and Theranostics. Molecules 2019, 24, 49. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010049
Franco Machado J, Silva RD, Melo R, G. Correia JD. Less Exploited GPCRs in Precision Medicine: Targets for Molecular Imaging and Theranostics. Molecules. 2019; 24(1):49. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010049
Chicago/Turabian StyleFranco Machado, João, Rúben D. Silva, Rita Melo, and João D. G. Correia. 2019. "Less Exploited GPCRs in Precision Medicine: Targets for Molecular Imaging and Theranostics" Molecules 24, no. 1: 49. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010049






