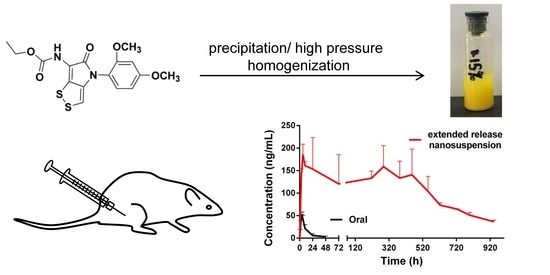
Optimization of Extended-Release ZL-004 Nanosuspensions for In Vivo Pharmacokinetic Study to Enhance Low Solubility and Compliance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation
2.1.1. Precipitation Method
2.1.2. High-Pressure Homogenization Method
2.2. In Vitro Release Study
2.3. Oral Administration
2.4. In Vivo Pharmacokinetic Study with Different Particle Sizes
2.5. In Vivo Pharmacokinetic Study with Different Administration Volume
2.6. Comparisons of Nanosuspensions Prepared by the Precipitation Method and the High-Pressure Homogenization Method
2.7. Physical Stability
3. Materials and Methods
3.1. Materials
3.2. Method
3.2.1. Preparation
Precipitation Method
High-Pressure Homogenization Method
3.2.2. Characterization
DSC
XRPD
SEM
Particle Size and Zeta Potential
3.2.3. In Vitro Release Study
3.2.4. In Vivo Pharmacokinetic Study
3.2.5. Physical Stability
3.2.6. The Content of Dissolved Drug
3.2.7. Significance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, C.; Sun, Y.; Wang, G.; Tan, X. Synthesis of Dithiolopyrrolone Derivatives and Their Leukocyte-Increasing Activities. Bull. Korean Chem. Soc. 2014, 35, 3489–3494. [Google Scholar] [CrossRef] [Green Version]
- Celmer, W.D.; Solomons, I.A. The Structures of Thiolutin and Aureothricin, Antibiotics Containing a Unique Pyrrolinonodithiole Nucleus. J. Am. Chem. Soc. 1955, 77, 2861–2865. [Google Scholar] [CrossRef]
- Li, B.; Wever, W.J.; Walsh, C.T.; Bowers, A.A. Dithiolopyrrolones: Biosynthesis, synthesis, and activity of a unique class of disulfide-containing antibiotics. Nat. Prod. Rep. 2014, 31, 905–923. [Google Scholar] [CrossRef] [PubMed]
- Liras, P. Holomycin, a dithiolopyrrolone compound produced by Streptomyces clavuligerus. Appl. Microbiol. Biotechnol. 2014, 98, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, C.; Yu, Z.; Wang, P.; Nian, S.; Deng, Y.; Wu, W.; Wang, G. Synthesis of Substituted 6-Amino-4-(2,4-dimethoxyphenyl)-[1,2]dithiolo[4,3-b]pyrrol-5-ones and Their Raising Leukocyte Count Activities. Chem. Pharm. Bull. 2013, 61, 351–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronchud, M.H.; Scarffe, J.H.; Thatcher, N.; Crowther, D.; Souza, L.M.; Alton, N.K.; Testa, N.G.; Dexter, T.M. Phase I/II study of recombinant human granulocyte colony-stimulating factor in patients receiving intensive chemotherapy for small cell lung cancer. Br. J. Cancer 1987, 56, 809–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinstler, O.B.; Brems, D.N.; Lauren, S.L.; Paige, A.G.; Hamburger, J.B.; Treuheit, M.J. Characterization and Stability of N-terminally PEGylated rhG-CSF. Pharm. Res. 1996, 13, 996–997. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Han, X.; Wang, L.; Du, P.; Yao, J.; Wu, D.; Song, Y.; Zhang, S.; Tang, L.; Shi, Y. A phase I study of different doses and frequencies of pegylated recombinant human granulocyte-colony stimulating factor (PEG rhG-CSF) in patients with standard-dose chemotherapy-induced neutropenia. Chin. J. Cancer Res. 2017, 29, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.M. Converting oral to long-acting injectable antipsychotics: A guide for the perplexed. CNS Spectr. 2017, 22, 14–28. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.M.; Liversidge, G.G. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef]
- Gora, S.; Mustafa, G.; Sahni, J.K.; Ali, J.; Baboota, S. Nanosizing of valsartan by high pressure homogenization to produce dissolution enhanced nanosuspension: Pharmacokinetics and pharmacodyanamic study. Drug Deliv. 2016, 23, 940–950. [Google Scholar] [CrossRef]
- Gopal, S.; Gassmann-Mayer, C.; Palumbo, J.; Samtani, M.N.; Shiwach, R.; Alphs, L. Practical guidance for dosing and switching paliperidone palmitate treatment in patients with schizophrenia. Curr. Med. Res. Opin. 2009, 26, 377–387. [Google Scholar] [CrossRef]
- Kumar, B.S.; Saraswathi, R.; Kumar, K.V.; Jha, S.K.; Venkates, D.P.; Dhanaraj, S.A. Development and characterization of lecithin stabilized glibenclamide nanocrystals for enhanced solubility and drug delivery. Drug Deliv. 2014, 21, 173–184. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X. Drug nanocrystals: In vivo performances. J. Control. Release 2012, 160, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Leng, D.; Chen, H.; Li, G.; Guo, M.; Zhu, Z.; Xu, L.; Wang, Y. Development and comparison of intramuscularly long-acting paliperidone palmitate nanosuspensions with different particle size. Int. J. Pharm. 2014, 472, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Shen, G.; Wang, D.; Pang, L.; Chen, Z.; Liu, Z. Development and characterization of glimepiride nanocrystal formulation and evaluation of its pharmacokinetic in rats. Drug Deliv. 2013, 20, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, B.; Muller, R.H.; Moschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.; York, P.; Blagden, N. Preparation of hydrocortisone nanosuspension through a bottom-up nanoprecipitation technique using microfluidic reactors. Int. J. Pharm. 2009, 375, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Muller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef]
- Van Klooster, G.; Hoeben, E.; Borghys, H.; Looszova, A.; Bouche, M.P.; van Velsen, F.; Baert, L. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob. Agents Chemother. 2010, 54, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Halbert, G.W. Modern Pharmaceutics, 5th ed.; Florence, A.T., Siepmann, J., Eds.; Informa Healthcare: New York, NY, USA, 2009. [Google Scholar]
- Zhao, F.; Luan, H.; Yang, L.; Ma, Y.; Zhang, X.; Zhang, S.; Wang, H. Preparation of Nanoparticles of Zl-004 and Preliminary Study on Its Pharmacokinetics. Chin. Pharm. J. 2013, 48, 2026–2033. [Google Scholar]
- Shen, J.; Burgess, D.J. In vitro–in vivo correlation for complex non-oral drug products: Where do we stand? J. Control. Release 2015, 219, 644–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cresswell, P.; Paquette, S.M.; Hickey, M.; Perkin, K.; Smith, G.; Liversidge, E.; Steinberg, B.; Manser, D.; Turncliff, R.; Palmieri, M.; et al. Aripiprazole Prodrug Compositions. U.S. Patent 20,160,045,495, 2016. [Google Scholar]
- Bishara, D. Once-monthly paliperidone injection for the treatment of schizophrenia. Neuropsychiatr. Dis. Treat. 2010, 6, 561–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turncliff, R.; Hard, M.; Du, Y.; Risinger, R.; Ehrich, E.W. Relative bioavailability and safety of aripiprazole lauroxil, a novel once-monthly, long-acting injectable atypical antipsychotic, following deltoid and gluteal administration in adult subjects with schizophrenia. Schizophr. Res. 2014, 159, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Boistelle, R.; Astier, J.P. Crystallization Mechanisms in Solution. J. Cryst. Growth 1988, 90, 14–30. [Google Scholar] [CrossRef]
- Ostwald, W. Studien über die Bildung und Umwandlung fester Körper. Z. Phys. Chem. 1897, 22U, 289–330. [Google Scholar] [CrossRef]
- Ely, D.R.; Edwin García, R.; Thommes, M. Ostwald–Freundlich diffusion-limited dissolution kinetics of nanoparticles. Powder Technol. 2014, 257, 120–123. [Google Scholar] [CrossRef]
- Nutan, M.T.H.; Reddy, I.K. Pharmaceutical Suspensions; Kulshreshtha, A.K., Singh, O.N., Wall, G.M., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Gregory, J.; Barany, S. Adsorption and flocculation by polymers and polymer mixtures. Adv. Colloid Interface Sci. 2011, 169, 1–12. [Google Scholar] [CrossRef]
- Shete, G.; Jain, H.; Punj, D.; Prajapat, H.; Akotiyaa, P.; Bansal, A.K. Stabilizers used in nano-crystal based drug delivery systems. J. Excipients Food Chem. 2014, 5, 184–208. [Google Scholar]
- Al Shaal, L.; Shegokar, R.; Muller, R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. 2011, 420, 133–140. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Formulation | D10 (nm) | D50 (nm) | D90 (nm) |
|---|---|---|---|
| NS-PA | 284 ± 27 | 424 ± 55 | 598 ± 98 |
| NS-PB | 459 ± 13 | 812 ± 48 | 1647 ± 166 |
| NS-PC | 645 ± 6 | 1185 ± 36 | 2249 ± 201 |
| NS-Hα | 591 ± 17 | 1134 ± 33 | 2518 ± 150 |
| Formulation | Preparation a | Concentration (mg/mL) | D10 (nm) | D50 (nm) | D90 (nm) |
|---|---|---|---|---|---|
| NS-PA-L | freeze drying | 6 | 274 | 397 | 558 |
| NS-PB-L | freeze drying | 6 | 472 | 836 | 1395 |
| NS-PC-L | freeze drying | 6 | 598 | 1156 | 2262 |
| NS-PB-S | concentrating, freeze drying | 30 | 448 | 806 | 1612 |
| NS-PC-S | concentrating, freeze drying | 30 | 618 | 1225 | 2466 |
| NS-Hα-1 | none | 30 | 624 | 1211 | 2701 |
| NS-Hα-2 | adding 0.15% PEG 4000 | 30 | 627 | 1144 | 2402 |
| Formulation | Tmax (0–72 h) (h) | Cmax (0–72 h) (ng/mL) | AUC0→t (µg·h/mL) | t1/2 (h) |
|---|---|---|---|---|
| Oral | 4.9 ± 0.8 | 48.6 ± 12.1 d | 0.6 ± 0.2 d | 11.0 ± 1.9 d |
| NS-PA-L | 14.5 ± 0.0 | 1104.7 ± 162.5 a | 192.3 ± 21.8 a | 170.5 ± 16.7 a |
| NS-PB-L | 14.5 ± 0.0 | 518.9 ± 109.7 a,†,b | 153.2 ± 20.2 a,b | 222.0 ± 23.3 a,b |
| NS-PC-L | 14.5 ± 0.0 | 685.5 ± 60.9 †,c | 163.0 ± 29.7 c | 406.8 ± 34.2 b |
| NS-PB-S | 7.0 ± 2.0 | 189.4 ± 32.5 b | 98.5 ± 21.2 b | 208.8 ± 17.4 † |
| NS-PC-S | 9.0 ± 2.0 | 232.9 ± 122.2 c | 110.2 ± 32.1 c | 266.9 ± 44.4 † |
| NS-Hα-1 | 6.0 ± 0.0 | 218.5 ± 31.9 | 113.1 ± 14.5 | 241.5 ± 39.2 |
| NS-Hα-2 | 7.0 ± 2.0 | 209.7 ± 24.2 d | 96.9 ± 9.8 d | 252.3 ± 48.8 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Chen, Y.; Zhu, J.; Wang, J.; Xu, Y.; Luan, H.; Wang, H. Optimization of Extended-Release ZL-004 Nanosuspensions for In Vivo Pharmacokinetic Study to Enhance Low Solubility and Compliance. Molecules 2019, 24, 7. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010007
Guo C, Chen Y, Zhu J, Wang J, Xu Y, Luan H, Wang H. Optimization of Extended-Release ZL-004 Nanosuspensions for In Vivo Pharmacokinetic Study to Enhance Low Solubility and Compliance. Molecules. 2019; 24(1):7. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010007
Chicago/Turabian StyleGuo, Chengyue, Yanna Chen, Junzhe Zhu, Jiaxin Wang, Ying Xu, Hansen Luan, and Hao Wang. 2019. "Optimization of Extended-Release ZL-004 Nanosuspensions for In Vivo Pharmacokinetic Study to Enhance Low Solubility and Compliance" Molecules 24, no. 1: 7. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010007