The Influence of Graft Length and Density on Dispersion, Crystallisation and Rheology of Poly(ε-caprolactone)/Silica Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Characterisation Methods
3. Results and Discussion
3.1. Grafting Reactions
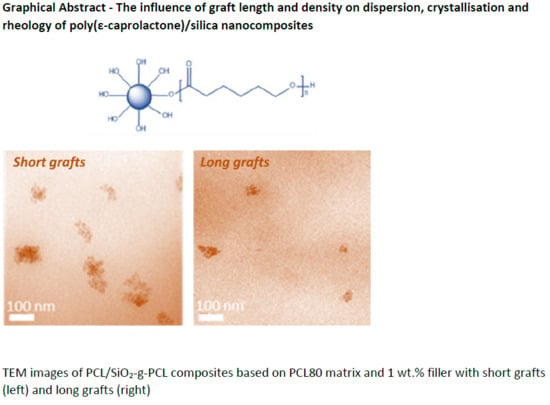
3.2. Dispersion and Morphology
3.3. Crystallisation Behaviour
3.3.1. Non-Isothermal Crystallization
3.3.2. Isothermal Crystallisation
3.4. Rheological Behaviour
3.4.1. Storage, Loss Modulus and Viscosity
3.4.2. Shear-Induced Crystallisation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schadler, L.S. Polymer-Based and Polymer-Filled Nanocomposites. In Nanocomposite Science and Technology, 1st ed.; Ajayan, P.M., Schadler, L.S., Braun, P.V., Eds.; Wiley-VCH: Weinheim, Germany, 2003; pp. 77–153. [Google Scholar]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Ou, Y.; Yang, F.; Yu, Z.Z. A new conception on the toughness of nylon 6/silica nanocomposite prepared via in situ polymerization. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 789–795. [Google Scholar] [CrossRef]
- Zhang, R.; Dowden, A.; Deng, H.; Baxendale, M.; Peijs, T. Conductive network formation in the melt of carbon nanotube/thermoplastic polyurethane composite. Compos. Sci. Technol. 2009, 69, 1499–1504. [Google Scholar] [CrossRef]
- Bilotti, E.; Zhang, R.; Deng, H.; Baxendale, M.; Peijs, T. Fabrication and property prediction of conductive and strain sensing TPU/CNT nanocomposite fibres. J. Mater. Chem. 2010, 20, 9449–9455. [Google Scholar] [CrossRef]
- Deng, H.; Skipa, T.; Bilotti, E.; Zhang, R.; Lellinger, D.; Mezzo, L.; Fu, Q.; Alig, I.; Peijs, T. Preparation of high-performance conductive polymer fibers through morphological control of networks formed by nanofillers. Adv. Funct. Mater. 2010, 20, 1424–1432. [Google Scholar] [CrossRef]
- Wang, X.; Foltz, V.J.; Rackaitis, M.; Böhm, G.G.A. Dispersing hairy nanoparticles in polymer melts. Polymer 2008, 49, 5683–5691. [Google Scholar] [CrossRef]
- Akcora, P.; Liu, H.; Kumar, S.K.; Moll, J.; Li, Y.; Benicewicz, B.C.; Schadler, L.S.; Acehan, D.; Panagiotopoulos, A.Z.; Pryamitsyn, V.; et al. Anisotropic self-assembly of spherical polymer-grafted nanoparticles. Nat. Mater. 2009, 8, 355–359. [Google Scholar] [CrossRef]
- Oberdisse, J. Aggregation of colloidal nanoparticles in polymer matrices. Soft Matter 2006, 2, 29–36. [Google Scholar] [CrossRef]
- Akcora, P.; Kumar, S.K.; Garcia Sakai, V.; Li, Y.; Benicewicz, B.C.; Schadler, L.S. Segmental dynamics in PMMA—Grafted nanoparticle composites. Macromolecules 2010, 43, 8275–8281. [Google Scholar] [CrossRef]
- Chevigny, C.; Dalmas, F.; Di Cola, E.; Gigmes, D.; Bertin, D.; Boue, F.; Jestin, J. Polymer-grafted-nanoparticles nanocomposites: Dispersion, grafted chain conformation, and rheological behavior. Macromolecules 2011, 44, 122–133. [Google Scholar] [CrossRef]
- Oh, H.; Green, P.F. Polymer chain dynamics and glass transition in athermal polymer/nanoparticle mixtures. Nat. Mater. 2008, 8, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ke, Y. Preparation of silica–PS composite particles and their application in PET. Eur. Polym. J. 2006, 42, 274–285. [Google Scholar]
- Rong, M.Z.; Zhang, M.Q.; Pan, S.L.; Lehmann, B.; Friedrich, K. Analysis of the interfacial interactions in polypropylene/silica nanocomposites. Polym. Int. 2004, 53, 176–183. [Google Scholar] [CrossRef]
- Hasegawa, R.; Aoki, Y.; Doi, M. Optimum graft density for dispersing particles in polymer melts. Macromolecules 1996, 29, 6656–6662. [Google Scholar] [CrossRef]
- McEwan, M.; Green, D. Rheological impacts of particle softness on wetted polymer-grafted silica nanoparticles in polymer melts. Soft Matter 2009, 5, 1705–1716. [Google Scholar] [CrossRef]
- Moon, J.H.; Ramaraj, B.; Lee, S.M.; Yoon, K.R.J. Direct grafting of ε-caprolactone on solid core/mesoporous shell silica spheres by surface-initiated ring-opening polymerization. J. Appl. Polym. Sci. 2008, 107, 2689–2694. [Google Scholar] [CrossRef]
- Joubert, M.; Delaite, C.; Bourgeat-Lami, E.; Dumas, P. Ring-opening polymerization of ε-caprolactone and L—Lactide from silica nanoparticles surface. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1976–1984. [Google Scholar] [CrossRef]
- Carrot, G.; Rutot-Houzé, D.; Pottier, A.; Degée, P.; Hilborn, J.; Dubois, P. Surface-initiated ring-opening polymerization: A versatile method for nanoparticle ordering. Macromolecules 2002, 35, 8400–8404. [Google Scholar] [CrossRef]
- Vassiliou, A.A.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Non-isothermal crystallisation kinetics of in situ prepared poly(ε-caprolactone)/surface-treated SiO2 nanocomposites. Macromol. Chem. Phys. 2007, 208, 364–376. [Google Scholar] [CrossRef]
- Avella, M.; Bondioli, F.; Cannillo, V.; Di Pace, E.; Errico, M.E.; Ferrari, A.M.; Focher, B.; Malinconico, M. Poly(ε-caprolactone) based nanocomposites: Influence of compatibilization on properties of poly(ε-caprolactone)/silica nanocomposites. Compos. Sci. Technol. 2006, 66, 886–894. [Google Scholar] [CrossRef]
- Zhou, B.; Tong, Z.Z.; Huang, J.; Xu, J.T.; Fan, Z.Q. Isothermal crystallization kinetics of multi-walled carbon nanotubes-graft-poly(ε-caprolactone) with high grafting degrees. Cryst. Eng. Comm. 2013, 15, 7824–7832. [Google Scholar] [CrossRef]
- L’Abee, R.M.A.; van Duin, M.; Goossens, J.G.P. Crystallization kinetics and crystalline morphology of poly(ε-caprolactone) in blends with grafted rubber particles. J. Polym. Sci. Part B Polym. Phys. 2011, 48, 1438–1448. [Google Scholar] [CrossRef]
- Eriksson, M.; Goossens, H.; Peijs, T. Influence of drying procedure on glass transition temperature of PMMA based nanocomposites. Nanocomposites 2015, 1, 36–45. [Google Scholar] [CrossRef]
- Avella, M.; Errico, M.E.; Rimedio, R.; Sadocco, P. Preparation of biodegradable polyesters/high-amylose-starch composites by reactive blending and their characterization. J. Appl. Polym. Sci. 2002, 83, 1432–1442. [Google Scholar] [CrossRef]
- Fillon, B.; Wittmann, J.C.; Lotz, B.; Thierry, A. Self-nucleation and recrystallization of isotactic polypropylene (α phase) investigated by differential scanning calorimetry. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1383–1993. [Google Scholar] [CrossRef]
- Fillon, B.; Lotz, B.; Thierry, A.; Wittmann, J.C. Self-nucleation and enhanced nucleation of polymers. Definition of a convenient calorimetric “efficiency scale” and evaluation of nucleating additives in isotactic polypropylene (α phase). J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1395–1405. [Google Scholar] [CrossRef]
- Eriksson, M.; Peijs, T.; Goossens, H. The effect of polymer molar mass and silica nanoparticles on the rheological and mechanical properties of poly(ε-caprolactone) nanocomposites. Nanocomposites 2018, 4, 112–126. [Google Scholar] [CrossRef]
- Eriksson, M.; Goffin, A.L.; Dubois, P.; Peijs, T.; Goossens, H. The influence of grafting on flow-induced crystallization and rheological properties of poly(ε-caprolactone)/cellulose nanocrystal nanocomposites. Nanocomposites 2018, 4, 87–101. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Muller, A.J. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to avoid common problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Chen, C.; Wang, Y.; Tang, H.; Li, Z.-M.; Hsiao, B.S. Graphene nanosheets and shear flow induced crystallization in isotactic polypropylene nanocomposites. Macromolecules 2011, 44, 2808–2818. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhong, G.L.; Lei, J.; Li, Z.M.; Hsiao, B.S. In situ synchrotron X-ray scattering study on isotactic polypropylene crystallization under the coexistence of shear flow and carbon nanotubes. Macromolecules 2011, 44, 8080–8092. [Google Scholar] [CrossRef]
- D’Haese, M.; Bart, G.; Van Puyvelde, P. The influence of calcium-stearate-coated calcium carbonate and talc on the quiescent and flow—Induced crystallization of isotactic poly(propylene). Macromol. Mater. Eng. 2011, 296, 603–616. [Google Scholar] [CrossRef]
- Naudy, S.; David, L.; Rochas, C.; Fulchiron, R. Shear induced crystallization of poly (m-xylylene adipamide) with and without nucleating additives. Polymer 2007, 48, 3273–3285. [Google Scholar] [CrossRef]















| Sample Code | Number of NH (groups/nm2) | Particles (g) | Catalyst (mg) | Caprolactone (g) | Toluene (g) | Time (h) |
|---|---|---|---|---|---|---|
| Short grafts | 0.5–0.7 | 1.1 | 100 | 6.0 | 80 | 16 |
| Medium grafts (half density) | 0.6–0.8 | 1.1 | 67 | 5.4 | 90 | 70 |
| Medium grafts | 0.5–0.8 | 1.1 | 122 | 9.9 | 90 | 70 |
| Long grafts | 0.5–0.8 | 1.0 | 118 | 8.1 | 95 | 72 |
| Particles | Silanized Particles | Short Grafts | Medium Grafts, Half Density | Medium Grafts | Long Grafts |
|---|---|---|---|---|---|
| Est. amount of grafts (% of silica particle weight) | 3.8 | 14.0 | 15.9 | 21.5 | 27.7 |
| Composite | Silica Content (wt%) | Tm (°C) | Tc (°C) | Crystallinity (%) | N.E. (%) |
|---|---|---|---|---|---|
| PCL40 | |||||
| PCL40 | 0 | 56.3 | 35.8 | 47.0 | 0.0 |
| No grafts | 1 | 56.7 | 35.8 | 44.8 | −0.3 |
| 3 | 56.4 | 36.9 | 45.4 | 11.6 | |
| 5 | 56.5 | 34.4 | 43.5 | −15.4 | |
| Short grafts | 1 | 56.6 | 36.3 | 47.9 | 5.5 |
| 3 | 57.0 | 36.5 | 46.2 | 7.3 | |
| 5 | 56.4 | 36.6 | 47.0 | 8.2 | |
| Medium grafts | 1 | 57.2 | 35.9 | 43.9 | 1.3 |
| 3 | 56.4 | 35.6 | 46.7 | −2.6 | |
| 5 | 56.4 | 36.2 | 46.3 | 4.1 | |
| Medium grafts, half density | 1 | 56.4 | 34.5 | 45.8 | −14.6 |
| 3 | 56.7 | 35.4 | 45.5 | −5.0 | |
| 5 | 56.6 | 35.1 | 44.2 | −7.7 | |
| Long grafts | 1 | 56.3 | 35.8 | 47.6 | −0.7 |
| 3 | 56.3 | 35.4 | 45.4 | −4.2 | |
| PCL80 | |||||
| PCL80 | 1 | 56.3 | 36.4 | 40.8 | 0 |
| No grafts | 1 | 56.5 | 40.9 | 40.9 | 32.1 |
| Short grafts | 1 | 56.1 | 38.4 | 41.3 | 13.6 |
| Medium grafts | 1 | 56.8 | 41.2 | 39.5 | 22.5 |
| Medium grafts, Half density | 1 | 56.1 | 41.4 | 40.6 | 27.0 |
| Long grafts | 1 | 56.4 | 41.0 | 40.2 | 25.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eriksson, M.; Hamers, J.; Peijs, T.; Goossens, H. The Influence of Graft Length and Density on Dispersion, Crystallisation and Rheology of Poly(ε-caprolactone)/Silica Nanocomposites. Molecules 2019, 24, 2106. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24112106
Eriksson M, Hamers J, Peijs T, Goossens H. The Influence of Graft Length and Density on Dispersion, Crystallisation and Rheology of Poly(ε-caprolactone)/Silica Nanocomposites. Molecules. 2019; 24(11):2106. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24112106
Chicago/Turabian StyleEriksson, Maria, Joris Hamers, Ton Peijs, and Han Goossens. 2019. "The Influence of Graft Length and Density on Dispersion, Crystallisation and Rheology of Poly(ε-caprolactone)/Silica Nanocomposites" Molecules 24, no. 11: 2106. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24112106