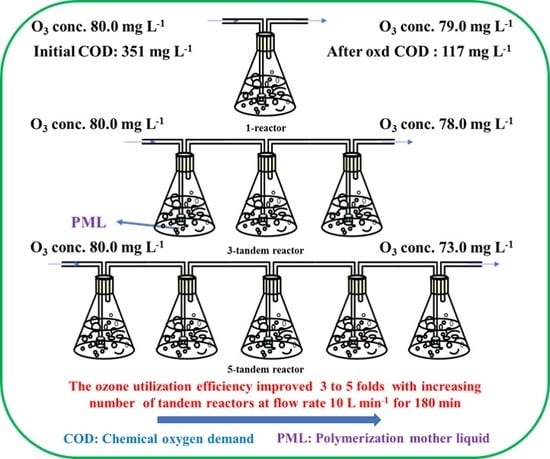
Improved Ozonation Efficiency for Polymerization Mother Liquid from Polyvinyl Chloride Production Using Tandem Reactors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Parameters Influencing COD Degradation
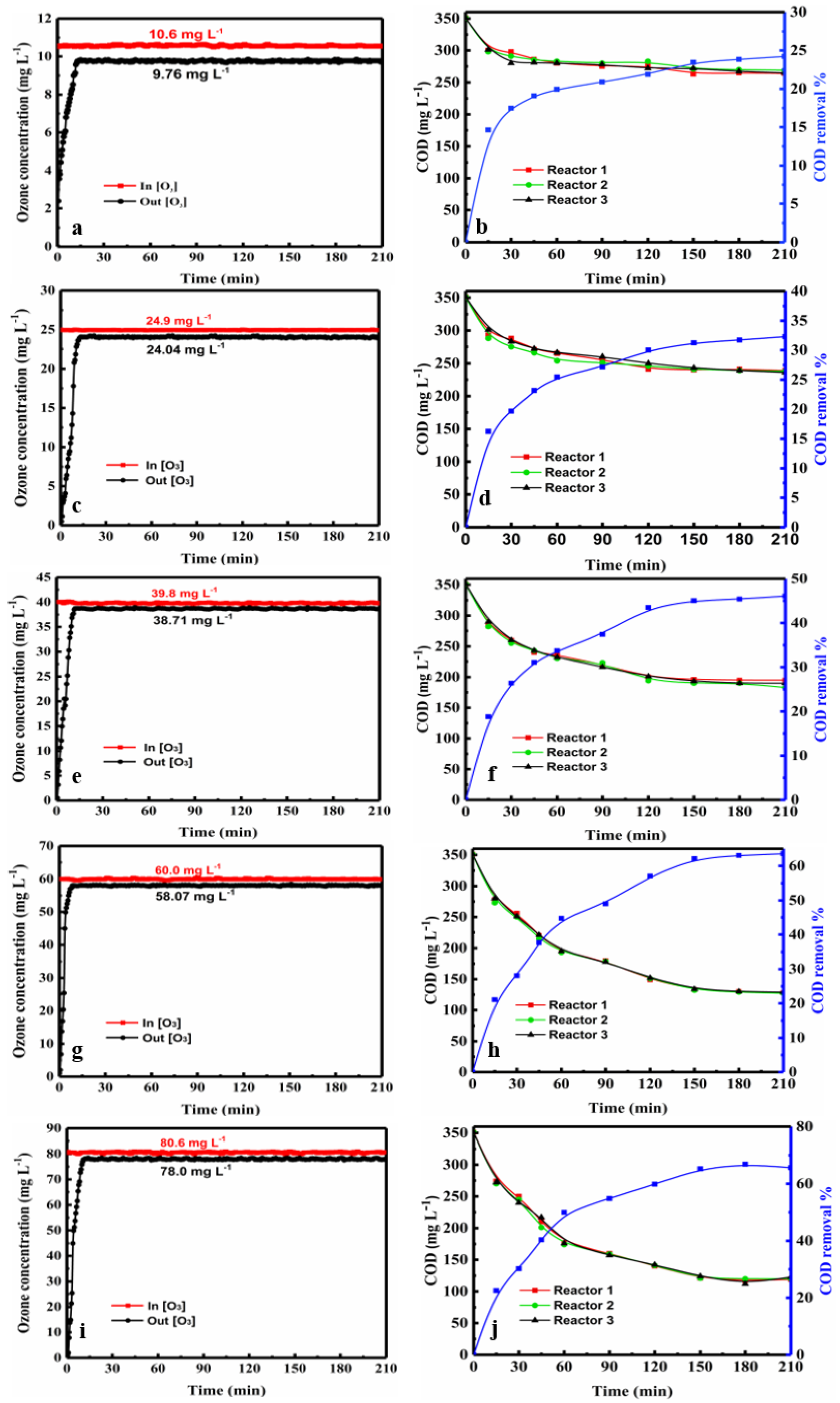
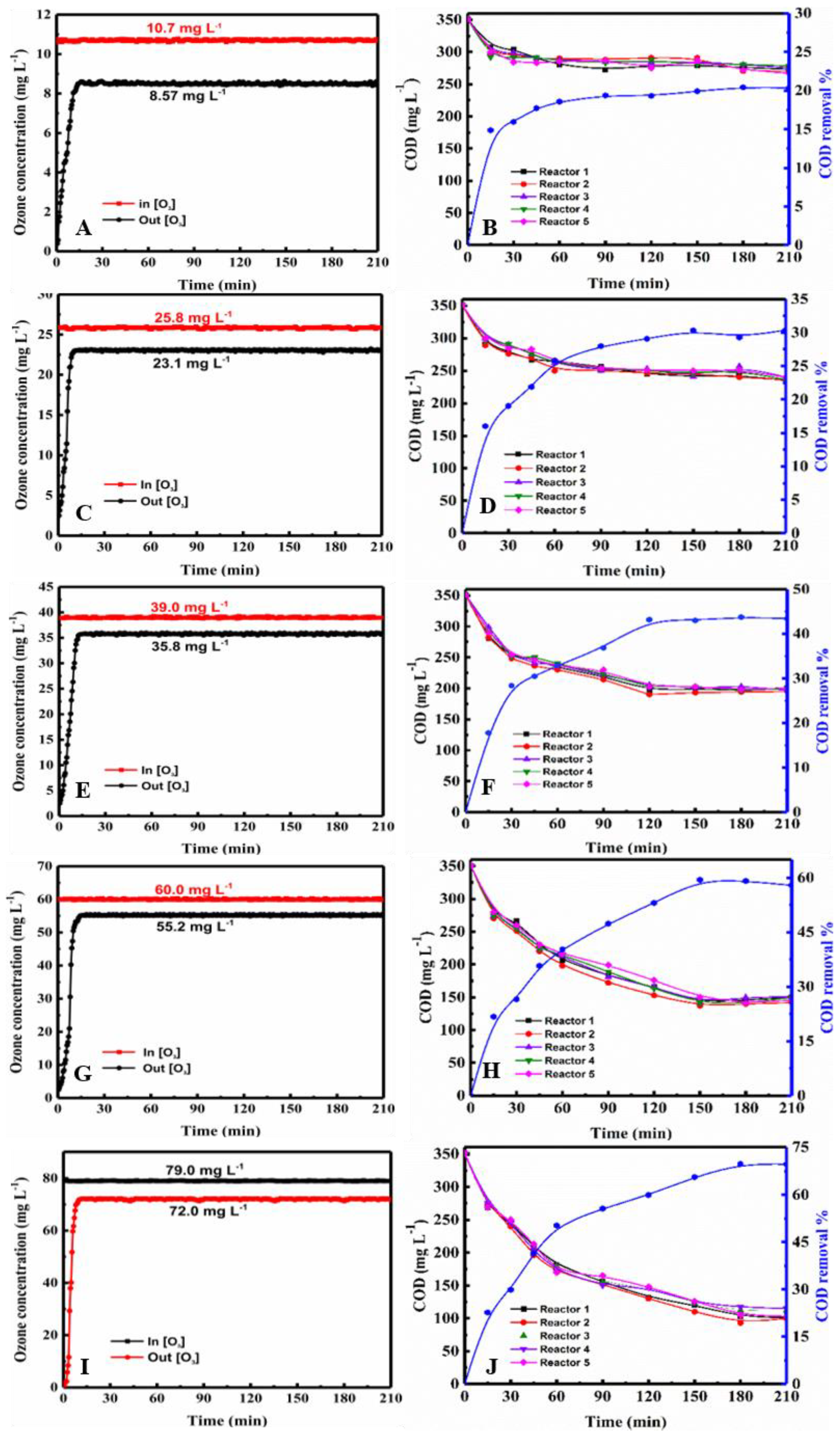
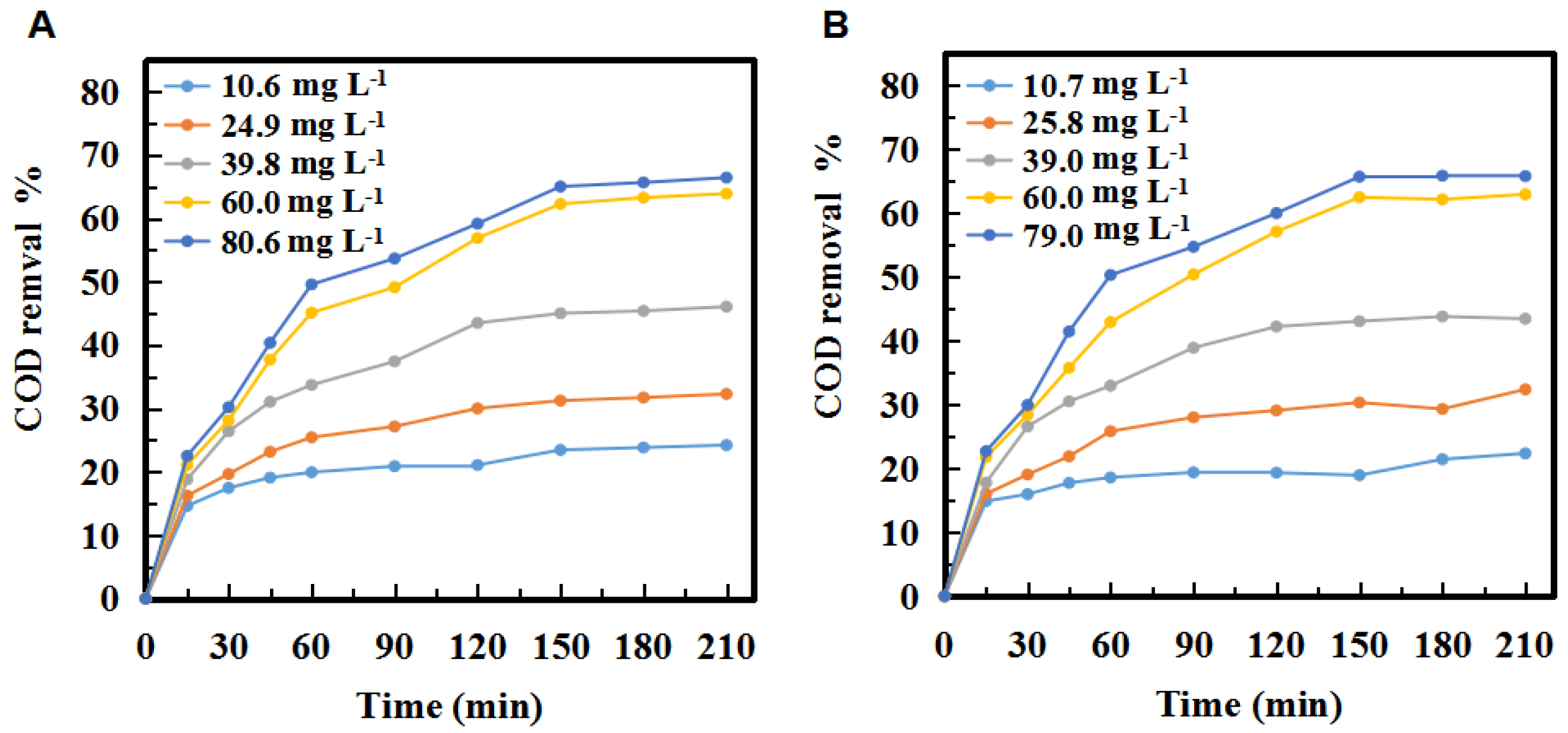
2.1.1. Effect of Ozone Concentration on COD
2.1.2. Effect of Ozonation Time
2.1.3. Effect of the Number of Reactors
2.1.4. PVA Degradation Pathway and Mechanism
2.2. Analysis of Ozone Utilization
2.2.1. Change in the Ozone Concentration in the Three-Tandem Reactors and Five-Tandem Reactors
2.2.2. Comparison the Ozone Consumption Ratio of the Three-Tandem Reactors and Five-Tandem Reactors
2.2.3. Ozone Utilization of the Three-Tandem Reactors and Five-Tandem Reactors
2.3. Biodegradability Analysis
3. Materials and Methods
3.1. PML Samples
3.2. Apparatus and Analysis Methods
3.3. COD Determination
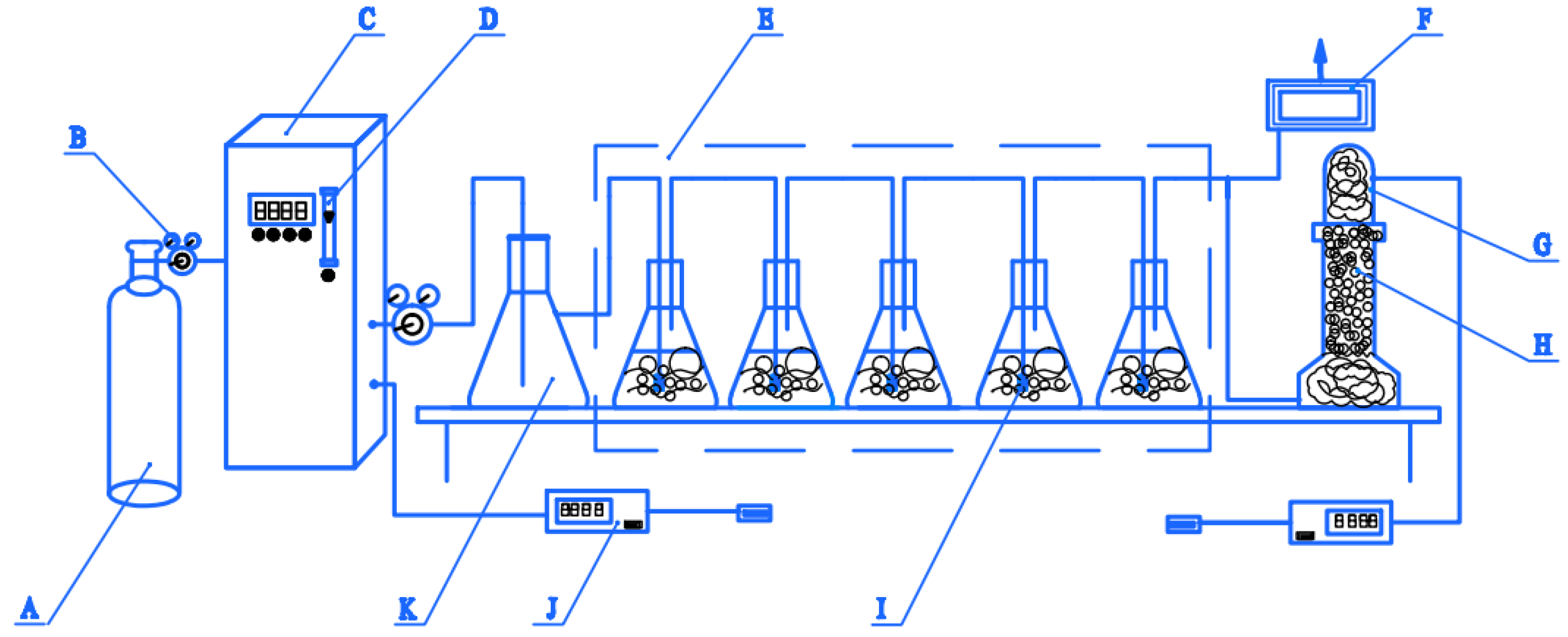
3.4. Experimental Set-Up and Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tacidelli, A.R.; Alves, J.J.N.; Vasconcelos, L.G.S.; Brito, R.P. Increasing PVC suspension polymerization productivity-An industrial application. Chem. Eng. Process. 2009, 48, 485–492. [Google Scholar] [CrossRef]
- Sun, Y.; Hua, X.; Ge, R.; Guo, A.; Guo, Z.; Dong, D.; Sun, W. Investigation on pretreatment of centrifugal mother liquid produced in the production of polyvinyl chloride by air-Fenton technique. Environ.Sci. Pollut. R. 2013, 20, 5797–5805. [Google Scholar] [CrossRef] [PubMed]
- Almubaddal, F.; Alrumaihi, K.; Ajbar, A. Performance optimization of coagulation/flocculation in the treatment of wastewater from a polyvinyl chloride plant. J. Hazard. Mater. 2009, 161, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Blanco, L.; Hermosilla, D.; Blanco, A.M.; Swinnen, N.; Prieto, D.; Negro, C. Assessment of the Performance of Membrane Bioreactors Applied to the Treatment of Industrial Effluents Containing Poly (vinyl alcohol). Ind. Eng. Chem. Res. 2015, 54, 5442–5449. [Google Scholar] [CrossRef]
- Huang, W.; Wang, L.; Zhou, W.; Lv, W.; Hu, M.; Chu, H.; Dong, B. Effects of combined ozone and PAC pretreatment on ultrafiltration membrane fouling control and mechanisms. J. Membrane. Sci. 2017, 533, 378–389. [Google Scholar] [CrossRef]
- Ying, S.; Aitong, G.; Rui, G. Pre-treatment of Centrifugal Mother Liquid in Polyvinyl Chloe-ride Production by Iron-Carbon Micro electrolysis technique. Environ. Sci. Technol. 2014, 37, 139–144. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.; Yan, J.; Wen, J.; Hu, Y.; Zhang, W. Intensive removal efficiency and mechanisms of carbon and ammonium in municipal wastewater treatment plant tail water by ozone oyster shells fix-bed bioreactor−membrane bioreactor combined system. Ecol. Eng. 2017, 101, 75–83. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Waindeskar, V.; Das, S.; Mudliar, S.N. Comparison of coagulation, ozone and ferrate treatment processes for color, COD and toxicity removal from complex textile wastewater. Water Sci. Technol. 2017, 76, 1001–1010. [Google Scholar] [CrossRef]
- Shah, A.D.; Dai, N.; Mitch, W.A. Application of ultraviolet, ozone, and advanced oxidation treatments to wash waters to destroy nitrosamines, Nitra mines, amines, and aldehydes formed during amine-based carbon capture. Environ. Sci. Technol. 2013, 47, 2799–2808. [Google Scholar] [CrossRef]
- Kermani, M.; Kakavandi, B.; Farzadkia, M.; Esrafili, A.; Jokandan, S.F.; Shahsavani, A. Catalytic ozonation of high concentrations of catechol over TiO2@Fe3O4 magnetic core-shell Nano catalyst: Optimization, toxicity and degradation pathway studies. J. Clean. Prod. 2018, 192, 597–607. [Google Scholar] [CrossRef]
- Maharaja, P.; Boopathy, R.; Karthikeyan, S.; Mahesh, M.; Komal, A.S.; Gupta, V.K.; Sekaran, G. Advanced oxidation of catechol in reverse osmosis concentrate generated in leather wastewater by Cu–graphite electrode. Int. J. Environ. Sci. Te. 2016, 13, 2143–2152. [Google Scholar] [CrossRef] [Green Version]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef] [PubMed]
- Takdastan, A.; Kakavandi, B.; Azizi, M.; Golshan, M. Efficient activation of peroxymonosulfate by using Ferro ferric oxide supported on carbon/UV/US system: A new approach into catalytic degradation of bisphenol A. Chem. Eng. J. 2018, 331, 729–743. [Google Scholar] [CrossRef]
- Bai, Z.; Yang, Q.; Wang, J. Catalytic ozonation of sulfamethazine using Ce0.1Fe0.9OOH as catalyst: Mineralization and catalytic mechanisms. Chem. Eng. J. 2016, 300, 169–176. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, C.; Zhang, D.; Li, L.; Pan, D. Heterogeneous catalytic ozonation of dibutyl phthalate in aqueous solution in the presence of iron-loaded activated carbon. Chemosphere 2015, 119, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Van Aken, P.; Van den Broeck, R.; Degrève, J.; Dewil, R. A pilot-scale coupling of ozonation and biodegradation of 2, 4-dichlorophenol-containing wastewater: The effect of biomass acclimation towards chlorophenol and intermediate ozonation products. J. Clean. Prod. 2017, 161, 1432–1441. [Google Scholar] [CrossRef]
- Feng, C.; Yang, L. Catalytic decomposition of analgin pharmaceutical wastewater in supercritical water. J. Adv. Oxid. Technol. 2015, 18, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Güçlü, D.; Şirin, N.; Şahinkaya, S.; Sevimli, M.F. Advanced treatment of coking wastewater by conventional and modified fenton processes. Environ. Prog. Sustain. 2013, 32, 176–180. [Google Scholar] [CrossRef]
- Han, D.; Kim, M.; Bae, W. Advanced treatment of recalcitrant textile wastewater and goal-oriented process design. KSCE J. Civ. Eng. 2014, 18, 86–92. [Google Scholar] [CrossRef]
- Hermosilla, D.; Merayo, N.; Gascó, A.; Blanco, Á. The application of advanced oxidation technologies to the treatment of effluents from the pulp and paper industry: A review. Environ. Sci. Pollut. R 2015, 22, 168–191. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A.; Ricco, G.; Mascolo, G.; Tiravanti, G.; Di Pinto, A.; Passino, R. Biodegradability enhancement of refractory pollutants by ozonation: A laboratory investigation on an azo-dyes intermediate. Water Sci. Technol. 1998, 38, 239–245. [Google Scholar] [CrossRef]
- Umar, M.; Roddick, F.; Fan, L.; Aziz, H.A. Application of ozone for the removal of bisphenol A from water and wastewater–a review. Chemosphere. 2013, 90, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Fanchiang, J.-M.; Tseng, D.-H. Degradation of anthraquinone dye C.I. Reactive Blue 19 in aqueous solution by ozonation. Chemosphere 2009, 77, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Hoigne, J.; Bader, H. The role of hydroxyl radical reactions in ozonation processes in aqueous solutions. Water Res. 1976, 10, 377–386. [Google Scholar] [CrossRef]
- Chelme-Ayala, P.; El-Din, M.G.; Smith, D.W. Kinetics and mechanism of the degradation of two pesticides in aqueous solutions by ozonation. Chemosphere 2010, 78, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Chen, G.; Ye, M.; Sun, S.; Ma, H.; Lin, W. Oxidation of potassium n-butyl xanthate with ozone: Products and pathways. J. Clean. Prod. 2016, 139, 287–294. [Google Scholar] [CrossRef]
- Aghapour, A.A.; Moussavi, G.; Yaghmaeian, K. Degradation and COD removal of catechol in wastewater using the catalytic ozonation process combined with the cyclic rotating-bed biological reactor. J. Environ. Manage. 2015, 157, 262–266. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, J.; Zhang, C. ZnAl2O4 as a novel high-surface-area ozonation catalyst: One-step green synthesis, catalytic performance and mechanism. Chem. Eng. J. 2015, 260, 623–630. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, J.; Yang, Q. Advanced treatment of municipal secondary effluent by catalytic ozonation using Fe3O4-CeO2/MWCNTs as efficient catalyst. Environ. Sci. Pollut. R 2017, 24, 9337–9349. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, J.; Yu, J.; Chen, J.; Chen, J. Catalytic ozonation for the degradation of acetylsalicylic acid in aqueous solution by magnetic CeO2 nanometer catalyst particles. Appl. Catal. B Environ. 2014, 144, 686–693. [Google Scholar] [CrossRef]
- Marce, M.; Domenjoud, B.; Esplugas, S.; Baig, S. Ozonation treatment of urban primary and biotreated wastewaters: Impacts and modeling. Chem. Eng. J. 2016, 283, 768–777. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, J.; Sun, Z.; Liu, H. Mechanism of heterogeneous catalytic ozonation of nitrobenzene in aqueous solution with modified ceramic honeycomb. Appl. Catal. B: Environ. 2009, 89, 326–334. [Google Scholar] [CrossRef]
- Hautaniemi, M.; Kallas, J.; Munter, R.; Trapido, M.; Laari, A. Modelling of Chlorophenol Treatment in Aqueous Solutions. 2. Ozonation under Basic Conditions. Ozone: Sci. Eng. 1998, 20, 283–302. [Google Scholar] [CrossRef]
- Hong, P.K.A.; Zeng, Y. Degradation of pentachlorophenol by ozonation and biodegradability of intermediates. Water Res. 2002, 36, 4243–4254. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B: Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Gottschak, C.; Libra, J.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Applications; John Wiley & Sons: Weinheim, Germany, 2010; pp. 50–63. [Google Scholar]
- Rice, R.G.; Netzer, A. Handbook of Ozone Technology and Applications; Ann Arbor Science: Ann Arbor, MI, USA, 1982; pp. 1–10. [Google Scholar]
- Lan, B.; Huang, R.; Li, L.; Yan, H.; Liao, G.; Wang, X.; Zhang, Q. Catalytic ozonation of p-chlorobenzoic acid in aqueous solution using Fe-MCM-41 as catalyst. Chem. Eng. J. 2013, 219, 346–354. [Google Scholar] [CrossRef]
- Huang, R.; Yan, H.; Li, L.; Deng, D.; Shu, Y.; Zhang, Q. Catalytic activity of Fe/SBA-15 for ozonation of dimethyl phthalate in aqueous solution. Appl. Catal. B: Environ. 2011, 106, 264–271. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Guo, X.; Guo, S.; Yan, G. Advanced ozone treatment of heavy oil refining wastewater by activated carbon supported iron oxide. J. Ind. Eng. Chem. 2014, 20, 2782–2791. [Google Scholar] [CrossRef]
- Huang, R.; Lan, B.; Chen, Z.; Yan, H.; Zhang, Q.; Bing, j.; Li, L. Catalytic ozonation of p-chlorobenzoic acid over MCM-41 and Fe loaded MCM-41. Chem. Eng. J. 2012, 180, 19–24. [Google Scholar] [CrossRef]
- Lü, X.; Zhang, Q.; Yang, W.; Li, X.; Zeng, L.; Li, L. Catalytic ozonation of 2,4-dichlorophenoxyacetic acid over novel Fe–Ni/AC. RSC Adv. 2015, 5, 10537–10545. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Efficient degradation of 2, 4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes. Chem. Engin. J. 2017, 320, 436–447. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Guo, Y.; Xu, D.; Gong, Y.; Tang, X.; Ma, H. Oxidative degradation of lurgi coal-gasification wastewater with Mn2O3, Co2O3, and CuO catalysts in supercritical water. Ind. Eng. Chem. Res. 2012, 51, 16573–16579. [Google Scholar] [CrossRef]
- Criegee, R. Mechanism of Ozonolysis. Angew. Chem. Int. Edit. Eng. 1975, 14, 745–752. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B: Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Beltrán, F.J.; García-Araya, J.F.; Álvarez, P.M. Wine Distillery Wastewater Degradation. 1. Oxidative Treatment Using Ozone and Its Effect on the Wastewater Biodegradability. J. Agr. Food Chem. 1999, 47, 3911–3918. [Google Scholar] [CrossRef]
- Ku, Y.; Su, W.-J.; Shen, Y.-S. Decomposition Kinetics of Ozone in Aqueous Solution. Ind. Eng. Chem. Res. 1996, 35, 3369–3374. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. Rate constants of reactions of ozone with organic and inorganic compounds in water—II: Dissociating organic compounds. Water Res. 1983, 17, 185–194. [Google Scholar] [CrossRef]
- Deleris, S.; Geaugey, V.; Camacho, P.; Debellefontaine, H.; Paul, E. Minimization of sludge production in biological processes: An alternative solution for the problem of sludge disposal. Water Sci. Technol. 2002, 46, 63–70. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Yu, H.-Q. Radiation-induced degradation of polyvinyl alcohol in aqueous solutions. Water Res. 2004, 38, 309–316. [Google Scholar] [CrossRef]
- Lei, L.; Hu, X.; Yue, P.L.; Bossmann, S.H.; Göb, S.; Braun, A.M. Oxidative degradation of poly vinyl alcohol by the photochemically enhanced Fenton reaction. J. Photoch. Photobio. A 1998, 116, 159–166. [Google Scholar] [CrossRef]
- Esplugas, S.; Giménez, J.; Contreras, S.; Pascual, E.; Rodríguez, M. Comparison of Different Advanced Oxidation Process for Phenol Degradation. Water Res. 2002, 36, 1034–1042. [Google Scholar] [CrossRef]
- Shu, H.-Y.; Chang, M.-C.; Fan, H.-J. Decolorization of azo dye acid black 1 by the UV/H2O2 process and optimization of operating parameters. J. Hazard. Mater. 2004, 113, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Kakavandi, B.; Jaafarzadeh, N.; Akbar Babaei, A. Catalytic ozonation of high saline petrochemical wastewater using PAC@FeIIFe2IIIO4: Optimization, mechanisms and biodegradability studies. Sep. Purif. Technol. 2017, 177, 293–303. [Google Scholar] [CrossRef]
- Zhuang, H.; Han, H.; Hou, B.; Jia, S.; Zhao, Q. Heterogeneous catalytic ozonation of biologically pretreated Lurgi coal gasification wastewater using sewage sludge based activated carbon supported manganese and ferric oxides as catalysts. Bioresour. Technol. 2014, 166, 178–186. [Google Scholar] [CrossRef]
- Saayman, G.B.; Schutte, C.F.; van Leeuwen, J. Chemical Control of Filamentous Sludge Bulking In A Full-Scale Biological Nutrient Removal Activated Sludge Plant. Ozone: Sci. Eng. 1998, 20, 1–15. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| 3-Reactor Device | 5-Reactor Device | ||||||
|---|---|---|---|---|---|---|---|
| Inlet O3 Conc. | Outlet O3 Conc. | ΔO3 Conc. | O3 Consumption Ratio % | Inlet O3 Conc. | Outlet O3 Conc. | ΔO3 Conc. | O3 Consumption Ratio % |
| 10.6 | 9.76 | 0.840 | 7.92 | 10.7 | 8.51 | 2.19 | 20.5 |
| 24.9 | 24.0 | 0.900 | 3.61 | 25.8 | 23.1 | 2.70 | 10.5 |
| 39.8 | 38.7 | 1.10 | 2.76 | 39.0 | 35.8 | 3.20 | 8.21 |
| 60.0 | 58.1 | 1.90 | 3.17 | 60.0 | 55.2 | 4.80 | 8.00 |
| 80.6 | 78.0 | 2.60 | 3.23 | 79.0 | 72.0 | 7.00 | 8.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Wang, P.; Zhang, Y.; Zan, X.; Zhu, W.; Jiang, Y.; Zhang, L.; Yasin, A. Improved Ozonation Efficiency for Polymerization Mother Liquid from Polyvinyl Chloride Production Using Tandem Reactors. Molecules 2019, 24, 4436. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24244436
Yang Z, Wang P, Zhang Y, Zan X, Zhu W, Jiang Y, Zhang L, Yasin A. Improved Ozonation Efficiency for Polymerization Mother Liquid from Polyvinyl Chloride Production Using Tandem Reactors. Molecules. 2019; 24(24):4436. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24244436
Chicago/Turabian StyleYang, Zhiyong, Penglei Wang, Yagang Zhang, Xingjie Zan, Wenjuan Zhu, Yingfang Jiang, Letao Zhang, and Akram Yasin. 2019. "Improved Ozonation Efficiency for Polymerization Mother Liquid from Polyvinyl Chloride Production Using Tandem Reactors" Molecules 24, no. 24: 4436. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24244436