Aromatic SNF-Approach to Fluorinated Phenyl tert-Butyl Nitroxides
Abstract
:1. Introduction
2. Results and Discussion
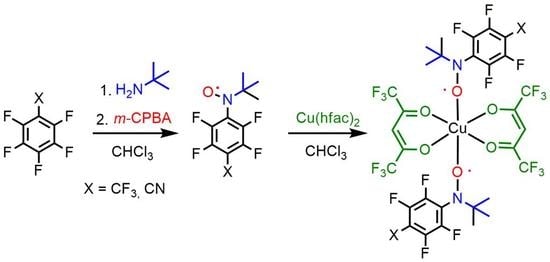
2.1. Synthesis of Fluorinated Nitoxodes 3a,b
2.2. ESR Measurement of Radicals 3a,b
2.3. Electrocemical Measurements
2.4. Crystal Structure of Radical 3a
2.5. Synthesis and Crystal Structure of Complexes [Cu(hfac)2(3a)2], [Cu(hfac)2(3b)2] and [Cu(hfac)2(2b)2]
2.6. Magnetic Measurements of Complexes [Cu(hfac)2(3a)2] and [Cu(hfac)2(3b)2]
3. Conclusions
4. Materials and Methods
4.1. Reagents and General Methods
4.2. Synthesis of 4-(tert-butylamino)arenes 2a,b (General Procedure)
4.3. Synthesis of 4-(N-tert-butyl(oxyl)amino)arenes 3a,b (General Procedure)
4.4. Synthesis of Complexes [Cu(hfac)2(3a)2], [Cu(hfac)2(3b)2] and [Cu(hfac)2(3b)2] (General Procedure)
4.5. X-Band ESR Measurements
4.6. Cyclic Voltammetry Measurements
4.7. Crystallographic Analysis
4.8. Thermal Analysis
4.9. Magnetic Measurements
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd Completely Revised and Enlarged ed.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Nenajdenko, V. (Ed.) Fluorine in Heterocyclic Chemistry; Springer: New York, NY, USA, 2014. [Google Scholar]
- Gakh, A.A.; Kirk, K.L. (Eds.) Fluorinated Heterocycles; ACS Symposium Series 1003; American Chemical Society: Washington, DC, USA, 2009. [Google Scholar]
- Reddy, V.P. Organofluorine Compounds in Biology and Medicine, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–23. [Google Scholar]
- Politanskaya, L.V.; Selivanova, G.A.; Panteleeva, E.V.; Tretyakov, E.V.; Platonov, V.E.; Nikul’shin, P.V.; Vinogradov, A.S.; Zonov, Ya.V.; Karpov, V.M.; Mezhenkova, T.V.; et al. Organofluorine chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2019, 88, 425–569. [Google Scholar] [CrossRef]
- Bobko, A.A.; Bagryanskaya, E.G.; Reznikov, V.A.; Kolosova, N.G.; Clanton, T.L.; Khramtsov, V.V. Redox-sensitive mechanism of NO scavenging by nitronyl nitroxides. Free Radical Bio. Med. 2004, 36, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Miyazaki, Y.; Inaba, A.; Paduan-Filho, A.; Bindilatti, V.; Oliveira, N.F., Jr.; Delen, Z.; Lahti, P.M. 2-(4,5,6,7-Tetrafluorobenzimidazol-2-yl)-4,4,5,5-tetramethyl-4,5-dihydro-1-H-imidazole-3-oxide- 1-oxyl, a hydrogen-bonded organic quasi-1D ferromagnet. J. Am. Chem. Soc. 2008, 130, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Delen, Z.; Lahti, P.M. Crystallography and magnetism of 1-(4-[N-tert-butylaminoxyl]- 2,3,5,6-tetrafluorophenyl)pyrrole. Polyhedron 2007, 26, 2031–2036. [Google Scholar] [CrossRef]
- Mei, H.; Han, J.; White, S.; Butler, G.; Soloshonok, V.A. Perfluoro-3-ethyl-2,4-dimethyl-3-pentyl persistent radical: A new reagent for direct, metal-free radical trifluoromethylation and polymer initiation. J. Fluor. Chem. 2019, 227, 109370. [Google Scholar] [CrossRef]
- Bannister, A.J.; Bricklebank, N.; Lavender, I.; Rawson, J.M.; Gregory, C.I.; Tanner, B.K.; Clegg, W.; Elsegood, M.R.J.; Palacio, F. Spontaneous magnetization in a sulfur-nitrogen radical at 36 K. Angew. Chem. Int. Ed. Engl. 1996, 35, 2533–2535. [Google Scholar] [CrossRef]
- Palacio, F.; Antorrena, G.; Castro, M.; Burriel, R.; Rawson, J.; Smith, J.N.B.; Bricklebank, N.; Novoa, J.; Ritter, C. High-Temperature Magnetic Ordering in a New Organic Magnet. Phys. Rev. Lett. 1997, 79, 2336–2339. [Google Scholar] [CrossRef] [Green Version]
- Constantinides, C.P.; Berezin, A.A.; Zissimou, G.A.; Manoli, M.; Leitus, G.M.; Bendikov, M.; Probert, M.R.; Rawson, J.M.; Koutentis, P.A. A Magnetostructural investigation of an abrupt spin transition for 1-Phenyl-3-trifluoromethyl-1,4-dihydrobenzo[e][1,2,4]triazin-4-yl. J. Am. Chem. Soc. 2014, 136, 11906–11909. [Google Scholar] [CrossRef]
- Zakrassov, A.; Shteiman, V.; Sheynin, Y.; Tumanskii, B.; Botoshansky, M.; Kapon, M.; Keren, A.; Kaftory, M.; Vos, T.E.; Miller, J.S. Synthesis, structural, and magnetic characterization of substituted benzimidazole-1-yl N,N′-dioxides. J. Mater. Chem. 2004, 14, 1827–1837. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Fedyushin, P.A.; Panteleeva, E.V.; Stass, D.V.; Bagryanskaya, I.Y.; Beregovaya, I.V.; Bogomyakov, A.S. Substitution of a Fluorine Atom in Perfluorobenzonitrile by a Lithiated Nitronyl Nitroxide. J. Org. Chem. 2017, 82, 4179–4185. [Google Scholar] [CrossRef]
- Fedyushin, P.; Panteleeva, E.; Bagryanskaya, I.; Maryunina, K.; Inoue, K.; Stass, D.; Tretyakov, E. An approach to fluorinated phthalonitriles containing a nitronyl nitroxide or iminonitroxide moiety. J. Fluor. Chem. 2019, 217, 1–7. [Google Scholar] [CrossRef]
- Fedyushin, P.; Gurskaya, L.; Panteleeva, E.; Koshcheev, B.; Maksimov, A.; Rybalova, T.V.; Zaytseva, E.; Tretyakov, E. Exploration of SNF-Approach toward Functionalized Nitronyl Nitroxides. Fluor. Notes 2019, 123, 7–8. [Google Scholar] [CrossRef]
- Iwamura, H.; Inoue, K.; Hayamizu, T. High-spin polynitroxide radicals as versatile bridging ligands for transition metal complexes with high ferri/ferromagnetic TC. Pure Appl. Chem. 1996, 68, 243–252. [Google Scholar] [CrossRef]
- Inoue, K.; Hayamizu, T.; Iwamura, H.; Hashizume, D.; Ohashi, Y. Assemblage and Alignment of the Spins of the Organic Trinitroxide Radical with a Quartet Ground State by Means of Complexation with Magnetic Metal Ions. A Molecule-Based Magnet with Three-Dimensional Structure and High TC of 46 K. J. Am. Chem. Soc. 1996, 118, 1803–1804. [Google Scholar] [CrossRef]
- Itoh, T.; Matsuda, K.; Iwamura, H.; Hori, K. Tris[p-(N-oxyl-N-tert-butylamino)phenyl]amine, -methyl, and -borane Have Doublet, Triplet, and Doublet Ground States, Respectively. J. Am. Chem. Soc. 2000, 122, 2567–2576. [Google Scholar] [CrossRef]
- Inoue, K.; Iwahori, F.; Markosyan, A.S.; Iwamura, H. Synthesis and magnetic properties of one-dimensional ferro- and ferrimagnetic chains made up of an alternating array of 1,3-bis(N-tert-butyl-N-oxyamino)benzene derivatives and Mn(II)(hfac)2. Coord. Chem. Rev. 2000, 198, 219–229. [Google Scholar] [CrossRef]
- Ferrer, J.R.; Lahti, P.M.; George, C.; Antorrena, G.; Palacio, F. Synthesis, Crystallography, and Magnetic Properties of 2-tert-Butylaminoxylbenzimidazole. Chem. Mater. 1999, 11, 2205–2210. [Google Scholar] [CrossRef]
- Lahti, P.M.; Ferrer, J.R.; George, C.; Oliete, P.; Julier, M.; Palacio, F. Hydrogen-bonded benzimidazole-based tert-butylnitroxides. Polyhedron 2001, 20, 1465–1473. [Google Scholar] [CrossRef]
- Ferrer, J.R.; Lahti, P.M.; George, C.; Oliete, P.; Julier, M.; Palacio, F. Role of Hydrogen Bonds in Benzimidazole-Based Organic Magnetic Materials: Crystal Scaffolding or Exchange Linkers. Chem. Mater. 2001, 13, 2447–2454. [Google Scholar] [CrossRef]
- Field, L.M.; Lahti, P.M. Coordination complexes of 1-(4-[N-tert-butyl-N-aminoxyl]phenyl)-1H-1,2,4-triazole with paramagnetic transition metal dications. Inorg. Chem. 2003, 42, 7447–7454. [Google Scholar] [CrossRef]
- Maspoch, D.; Catala, L.; Gerbier, P.; Ruiz-Molina, D.; Vidal-Gancedo, J.; Wurst, K.; Rovira, C.; Veciana, J. Radical para-benzoic acid derivatives: Transmission of ferromagnetic interactions through hydrogen bonds at long distances. Chem.–Eur. J. 2002, 8, 3635–3645. [Google Scholar] [CrossRef]
- Field, L.M.; Lahti, P.M. Magnetism of conjugated organic nitroxides: Structural scaffolding and exchange pathways. Chem. Mater. 2003, 15, 2861–2863. [Google Scholar] [CrossRef]
- Nagao, O.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Okada, K. Magnetic behavior of a manganese(II) complex of nitroxide-substituted 1,3-di(4-pyridyl)benzene. Polyhedron 2001, 20, 1653–1658. [Google Scholar] [CrossRef]
- Hicks, R.G. (Ed.) Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; John Wiley and Sons: Chichester, UK, 2010. [Google Scholar]
- Tretyakov, E.V.; Ovcharenko, V.I. The chemistry of nitroxide radicals in the molecular design of magnets. Russ. Chem. Rev. 2009, 78, 971–1012. [Google Scholar] [CrossRef]
- Goldman, J.; Petersen, T.E.; Torssell, K.; Becher, J. Fluorine-19 and proton NMR and ESR investigations of aryl tert-butyl nitroxides and nitronylnitroxides. Tetrahedron 1973, 29, 3833–3843. [Google Scholar] [CrossRef]
- Pedersen, J.A.; Torssell, K. Electron spin resonance and nuclear magnetic resonance spectra of sterically hindered aromatic nitroxide radicals. Synthesis of stable nitroxide radicals. Acta Chem. Scand. 1971, 25, 3151–3162. [Google Scholar] [CrossRef] [Green Version]
- Platonov, V.E.; Haas, A.; Schelvis, M.; Lieb, M.; Dvornikova, K.V.; Osina, O.I.; Gatilov, Yu.V. Polyfluorinated arylnitramines. J. Fluor. Chem. 2001, 109, 131–139. [Google Scholar] [CrossRef]
- Suga, T.; Pu, Y.-J.; Kasatori, S.; Nishide, H. Cathode- and Anode-Active Poly(nitroxylstyrene)s for Rechargeable Batteries: P- and n-Type Redox Switching via Substituent Effects. Macromolecules 2007, 40, 3167–3173. [Google Scholar] [CrossRef]
- Ohshita, J.; Iida, T.; Ohta, N.; Komaguchi, K.; Shiotani, M.; Kunai, A. Synthesis of Phenylnitroxides Bridged by an sp3-Linkage. Org. Lett. 2002, 4, 403–406. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular Nonbonded Contact Distances in Organic Crystal Structures: Comparison with Distances Expected from van der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Grand, A.; Laugier, J.; Pardi, L.; Rey, P. Moderate Ferromagnetic Exchange between Copper(II) and a Nitronyl Nitroxide in a Square-Pyramidal Adduct. MO Interpretation of the Mechanism of Exchange in Copper(II)–Nitroxide Complexes. Inorg. Chem. 1988, 27, 1031–1035. [Google Scholar] [CrossRef]
- Iwahori, F.; Markosyan, A.S.; Inoue, K. Structures and Magnetic Properties of the Complexes Made up by Cu(hfac)2 and Bisnitroxide Radical Derivatives. Mol. Cryst. Liq. Cryst. 2002, 376, 449–454. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Kitano, M.; Kumada, H.; Koga, N.; Iwamura, H. Regiospecificity in the Exchange Coupling of the Spins of Copper(II) Ion Coordinated with the Ring Nitrogen Atoms and N-tert-Butylaminoxyl Radical Attached as a Substituent on the Pyridine and N-Phenylimidazole Rings. Inorg. Chem. 1998, 37, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Field, L.M.; Lahti, P.M.; Palacio, F.; Paduan-Filho, A. Manganese(II) and Copper(II) Hexafluoroacetylacetonate 1:1 Complexes with 5-(4-[N-tert-Butyl-N-aminoxyl]phenyl)pyrimidine: Regiochemical Parity Analysis for Exchange Behavior of Complexes between Radicals and Paramagnetic Cations. J. Am. Chem. Soc. 2003, 125, 10110–10118. [Google Scholar] [CrossRef]
- Lanfranc de Panthou, F.; Luneau, D.; Musin, R.; Oehrstroem, L.; Grand, A.; Turek, P.; Rey, P. Spin-Transition and Ferromagnetic Interactions in Copper(II) Complexes of 2-(3-Pyridyl)imidazole N-Oxide. Dependence of the Magnetic Properties upon Crystal Packing. Inorg. Chem. 1996, 35, 3484–3491. [Google Scholar] [CrossRef]
- Musin, R.N.; Schastnev, P.V.; Malinovskaya, S.A. Delocalization mechanism of ferromagnetic exchange interactions in complexes of copper(II) with nitroxyl radicals. Inorg. Chem. 1992, 31, 4118–4121. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Fokin, S.V.; Romanenko, G.V.; Shvedenkov, Yu.G.; Ikorskii, V.N.; Tretyakov, E.V.; Vasilevskii, S.F. Nonclassical Spin Transitions. J. Struct. Chem. 2002, 43, 153–167. [Google Scholar] [CrossRef]
- Ovcharenko, V.; Bagryanskaya, E. Breathing Crystals from Copper Nitroxyl Complexes in Spin-Crossover Materials: Properties and Applications; Halcrow, M.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 239–280. [Google Scholar]
- Drozdyuk, I.Yu.; Tolstikov, S.E.; Tretyakov, E.V.; Veber, S.L.; Ovcharenko, V.I.; Sagdeev, R.Z.; Bagryanskaya, E.G.; Fedin, M.V. Light-Induced Magnetostructural Anomalies in a Polymer Chain Complex of Cu(hfac)2 with tert-Butylpyrazolylnitroxides. J. Phys. Chem. A 2013, 117, 6483–6488. [Google Scholar] [CrossRef]
- Kaszub, W.; Marino, A.; Lorenc, M.; Collet, E.; Bagryanskaya, E.G.; Tretyakov, E.V.; Ovcharenko, V.I.; Fedin, M.V. Ultrafast Photoswitching in a Copper-Nitroxide-Based Molecular Magnet. Angew. Chem. Int. Ed. 2014, 53, 10636–10640. [Google Scholar] [CrossRef]
- Barskaya, I.Yu.; Tretyakov, E.V.; Sagdeev, R.Z.; Ovcharenko, V.I.; Bagryanskaya, E.G.; Maryunina, K.Y.; Takui, T.; Sato, K.; Fedin, M.V. Photoswitching of a Thermally Unswitchable Molecular Magnet Cu(hfac)2Li-Pr Evidenced by Steady-State and Time-Resolved Electron Paramagnetic Resonance. J. Am. Chem. Soc. 2014, 136, 10132–10138. [Google Scholar] [CrossRef] [Green Version]
- Barskaya, I.Yu.; Veber, S.L.; Fokin, S.V.; Tretyakov, E.V.; Bagryanskaya, E.G.; Ovcharenko, V.I.; Fedin, M.V. Structural Specifics of Light-Induced Metastable States in Copper(II)-Nitroxide Molecular Magnets. Dalton Trans. 2015, 44, 20883–20888. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Li, Y.-X.; Yang, S.-L.; Zhang, C.-X.; Wang, Q.-L. Two copper complexes based on nitronyl nitroxide with different halides: Structures and magnetic properties. J. Coord. Chem. 2017, 70, 487–496. [Google Scholar]
- SADABS, v. 2008-1; Bruker AXS: Madison, WI, USA, 2008.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. PLATON, A Multipurpose Crystallographic Tool (Version, 10M); Utrecht University: Utrecht, The Netherlands, 2003. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds 3a,b, [Cu(hfac)2(3a)2], and [Cu(hfac)2(3b)2] are available from the authors. |








| Compound | E1/2ox, V | E1/2Red, V |
|---|---|---|
| 3a * | 1.04 | −1.44 |
| 3b * | 1.00 | −1.14 |
| [Cu(hfac)2(3a)2] * | 1.04 | −1.40 |
| [Cu(hfac)2(3b)2] * | 1.06 | −1.20 |
| 4a ** | 0.38 | −1.63 |
| 4b ** | 0.53 | −1.37 |
| Compound | 3a | [Cu(hfac)2(3a)2] | [Cu(hfac)2(3b)2] |
|---|---|---|---|
| Empirical formula | C11H9F7NO | C32H20CuF26N2O6 | C32H20CuF20N4O6 |
| Formula weight | 304.19 | 1086.04 | 1000.06 |
| Crystal system | Orthorhombic | Triclinic | Monoclinic |
| Space group | Pbca | P-1 | P21/c |
| a, Å | 11.6131(7) | 10.0680(3) | 11.3678(8) |
| b | 10.9047(7) | 13.8773(5) | 8.6913(7) |
| c | 19.7695(12) | 16.1651(6) | 20.219(1) |
| α, o | 90.00 | 96.289(2) | 90.00 |
| β, o | 90.00 | 102.367(2) | 90.158(3) |
| γ, o | 90.00 | 104.872(2) | 90.00 |
| Volume, Å3 | 2503.6(3) | 2099.6(1) | 1997.6(2) |
| Z | 8 | 2 | 2 |
| Density (calcd.), mg·m−3 | 1.614 | 1.718 | 1.663 |
| Abs. coefficient, mm−1 | 0.174 | 0.680 | 0.687 |
| F(000) | 1224 | 1074 | 994 |
| Crystal size, mm3 | 0.40 × 0.70 × 1.00 | 0.30 × 0.40 × 0.50 | 0.10 × 0.12 × 0.80 |
| Θ range for data collection, ° | 2.76–26.07 | 1.31–26.43 | 1.79–25.05 |
| Index ranges | −14 ≤ h ≤ 14, −13 ≤ k ≤ 13, −24 ≤ l ≤ 24 | −12 ≤ h ≤ 12, −17 ≤ k ≤ 17, −20 ≤ l ≤ 20 | −13 ≤ h ≤ 13, −10 ≤ k ≤ 10, −24 ≤ l ≤ 24 |
| Reflections collected | 34,642 | 64,762 | 26,173 |
| Independent reflections | 2473 R(int) 0.0266 | 8633 R(int) 0.052 | 3530 R(int) 0.069 |
| Completeness to θ 50º, % | 99.6 | 99.7 | 99.7 |
| Data/restraints/parameters | 2473/0/184 | 8633/0/613 | 3530/48/376 |
| Goodness-of-fit on F2 | 1.044 | 1.007 | 1.03 |
| Reflections with I > 2σ(I) | 2057 | 5535 | 2587 |
| Final R indices at I > 2σ(I) | R1 0.0555, wR2 0.1399 | R1 0.0630, wR2 0.1764 | R1 0.0530, wR2 0.1466 |
| Final R indices (all data) | R1 0.680, wR2 0.1554 | R1 0.1034, wR2 0.2165 | R1 0.0791, wR2 0.1716 |
| Largest diff. peak/hole, e·Å−3 | 0.59/−0.27 | 0.73/−0.47 | 0.58/−0.38 |
| Parameter | [Cu(hfac)2(3a)2] 1 | [Cu(hfac)2(3b)2] | |
|---|---|---|---|
| Bond Lengths, Å | |||
| Cu1–O1 | 2.411(5) | 2.398(4) | 2.452(4) |
| Cu1–O2 | 1.942(3) | 1.946(3) | 1.944(2) |
| Cu1–O3 | 1.935(3) | 1.935(3) | 1.937(2) |
| Bond angles, ° | |||
| O1–Cu1–O2 | 89.0(1) | 88.1(1) | 86.6(1) |
| O1–Cu1–O3 | 84.1(1) | 82.3(1) | 83.9(1) |
| O2–Cu1–O3 | 92.4(1) | 92.4(1) | 92.7(1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tretyakov, E.; Fedyushin, P.; Panteleeva, E.; Gurskaya, L.; Rybalova, T.; Bogomyakov, A.; Zaytseva, E.; Kazantsev, M.; Shundrina, I.; Ovcharenko, V. Aromatic SNF-Approach to Fluorinated Phenyl tert-Butyl Nitroxides. Molecules 2019, 24, 4493. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24244493
Tretyakov E, Fedyushin P, Panteleeva E, Gurskaya L, Rybalova T, Bogomyakov A, Zaytseva E, Kazantsev M, Shundrina I, Ovcharenko V. Aromatic SNF-Approach to Fluorinated Phenyl tert-Butyl Nitroxides. Molecules. 2019; 24(24):4493. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24244493
Chicago/Turabian StyleTretyakov, Evgeny, Pavel Fedyushin, Elena Panteleeva, Larisa Gurskaya, Tatyana Rybalova, Artem Bogomyakov, Elena Zaytseva, Maxim Kazantsev, Inna Shundrina, and Victor Ovcharenko. 2019. "Aromatic SNF-Approach to Fluorinated Phenyl tert-Butyl Nitroxides" Molecules 24, no. 24: 4493. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24244493