Structural and Rheological Properties of Pectins Extracted from Industrial Sugar Beet By-Products
Abstract
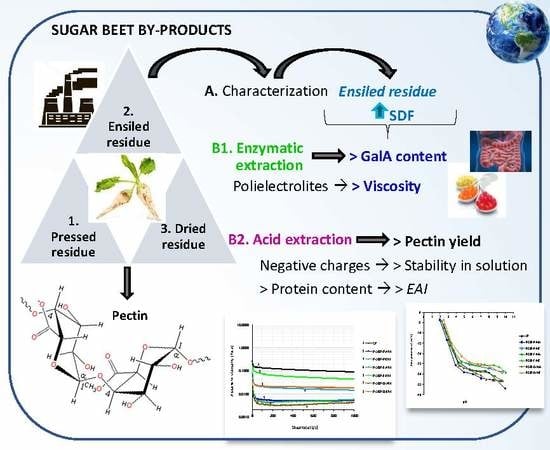
:1. Introduction
2. Results and Discussion
2.1. Overall Characterization of Sugar Beet By-Products
2.2. Pectin Extraction and Characterization
2.1.1. Yield, Monomeric Composition and Protein
2.1.2. Degree of Methoxylation (DM) and Molecular Weight (Mw)
2.1.3. Emulsifying Activity Index (EAI)
2.1.4. Zeta Potential (ζ) and Apparent Viscosity (η)
3. Materials and Methods
3.1. Samples
3.2. Physicochemical Characterization of Sugar Beet by-Products
3.3. Pectin Extraction
3.3.1. Acid Method
3.3.2. Enzymatic Method
3.4. Pectin Characterization
3.4.1. Monomeric Composition
3.4.2. Protein Content
3.4.3. Degree of Methylesterification (DM)
3.4.4. Molecular Weight (Mw)
3.4.5. Emulsifying Activity
3.4.6. Zeta potential (ζ)
3.4.7. Apparent Viscosity
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eurostat. Agriculture, Forestry and Fishery Statistics, 2017th ed.; Forti, R., Ed.; Imprimerie Centrale: Luxembourg, 2017; ISBN 978-92-79-63350-8. [Google Scholar]
- RedCorn, R.; Fatemi, S.; Engelberth, A.S. Comparing end-Use potential for industrial food-Waste sources. Engineering 2018, 4, 371–380. [Google Scholar] [CrossRef]
- Maravić, N.; Šereš, Z.; Vidović, S.; Mišan, A.; Milovanović, I.; Radosavljević, R.; Pavlić, B. Subcritical water hydrolysis of sugar beet pulp towards production of monosaccharide fraction. Ind. Crops Prod. 2018, 115, 32–39. [Google Scholar] [CrossRef]
- Agoda-Tandjawa, G.; Durand, S.; Gaillard, C.; Garnier, C.; Doublier, J.L. Properties of cellulose/pectins composites: Implication for structural and mechanical properties of cell wall. Carbohydr. Polym. 2012, 90, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Sousa, A.G.; Crépeau, M.-J.; Sorensen, S.O.; Ralet, M.-C. Characterization of citrus pectin samples extracted under different conditions: Influence of acid type and pH of extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Lazarte, A.; Kachrimanidou, V.; Villamiel, M.; Rastall, R.A.; Moreno, F.J. In vitro fermentation properties of pectins and enzymatic-modified pectins obtained from different renewable bioresources. Carbohydr. Polym. 2018, 199, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Jankovská, P.; Čopíková, J.; Sinitsya, A. The determination of ferulic acid in sugar beet pulp. Czech J. Food Sci. 2001, 19, 143–147. [Google Scholar] [CrossRef]
- Levigne, S.; Ralet, M.C.; Thibault, J.F. Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydr. Polym. 2002, 49, 145–153. [Google Scholar] [CrossRef]
- Siew, C.K.; Williams, P.A. Role of protein and ferulic acid in the emulsification properties of sugar beet pectin. J. Agric. Food Chem. 2008, 56, 4164–4171. [Google Scholar] [CrossRef]
- Lv, C.; Wang, Y.; Wang, L.-J.; Li, D.; Adhikari, B. Optimization of production yield and functional properties of pectin extracted from sugar beet pulp. Carbohydr. Polym. 2013, 95, 233–240. [Google Scholar] [CrossRef]
- Guo, X.; Guo, X.; Meng, H.; Zhang, B.; Yu, S. Using the high temperature resistant pH electrode to auxiliarily study the sugar beet pectin extraction under different extraction conditions. Food Hydrocoll. 2017, 70, 105–113. [Google Scholar] [CrossRef]
- Huang, X.; Li, D.; Wang, L. Effect of particle size of sugar beet pulp on the extraction and property of pectin. J. Food Eng. 2018, 218, 44–49. [Google Scholar] [CrossRef]
- Álvarez, S.; Méndez, P.; Martínez-Fernández, A. Fermentative and nutritive quality of banana by-product silage for goats. J. Appl. Anim. Res. 2015, 43, 396–401. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Spears, J.W. Bioaccessibility of iron from soil is increased by silage fermentation. J. Dairy Sci. 2009, 92, 2896–2905. [Google Scholar] [CrossRef]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Yoo, J.; Ko, S.; Lee, S. Extraction and characterization of pectin from Yuza (Citrus junos) pomace: A comparison of conventional-chemical and combined physical-enzymatic extractions. Food Hydrocoll. 2012, 29, 160–165. [Google Scholar] [CrossRef]
- Zykwinska, A.; Boiffard, M.-H.; Kontkanen, H.; Buchert, J.; Thibault, J.-F.; Bonnin, E. Extraction of green labeled pectins and pectic oligosaccharides from plant byproducts. J. Agric. Food Chem. 2008, 56, 8926–8935. [Google Scholar] [CrossRef]
- Sun, B.; Duan, L.; Peng, G.; Li, X.; Xu, A. Efficient production of glucose by microwave-assisted acid hydrolysis of cellulose hydrogel. Bioresour. Technol. 2015, 192, 253–256. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Mayworm, M.A.S.; Buckeridge, M.S.; Salatino, A. Monomer composition of polysaccharides of seed cell walls and the taxonomy of the Vochysiaceae. Phytochemistry 2000, 55, 581–587. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Lillo, L.E.; Sáenz, C.; Urzúa, C.C.; Zárate, O. Chemical characterization of the mucilage from fruits of Opuntia ficus indica. Carbohydr. Polym. 2006, 63, 263–267. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Reactions of Sugars. In Food Carbohydrate Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons, Inc.: Chichester-West Sussex, UK, 2013; pp. 35–47. ISBN 9781118688496. [Google Scholar]
- FAO; WHO. Compendium of Food Additive Specifications, 7th ed.; Joint FAO/WHO Expert Committee on Food Additives; FAO: Rome, Italy, 2009; Volume 4, ISBN 9251055599. [Google Scholar]
- Shi, L.; Gunasekaran, S. Preparation of pectin-ZnO nanocomposite. Nanoscale Res. Lett. 2008, 3, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Nisar, T.; Liang, D.; Hou, Y.; Sun, L.; Guo, Y. Low methoxyl pectin gelation under alkaline conditions and its rheological properties: Using NaOH as a pH regulator. Food Hydrocoll. 2018, 79, 560–571. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Tamburino, A.; Carnaroglio, D.; Cravotto, G.; Ilharco, L.M.; Pagliaro, M. Controlling the degree of esterification of citrus pectin for demanding applications by selection of the source. ACS Omega 2017, 2, 7991–7995. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, D.; Wang, L. Characterization of pectin extracted from sugar beet pulp under different drying conditions. J. Food Eng. 2017, 211, 1–6. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, S.; Gan, J.; Liu, Y.; Zhu, Q.; Yin, L. New insights into the functionality of protein to the emulsifying properties of sugar beet pectin. Food Hydrocoll. 2016, 57, 262–270. [Google Scholar] [CrossRef]
- Cabra, V.; Arreguín, R.; Farres, A. Boletín de la Sociedad Química de México; Sociedad Química de México: Barranca del Muerto, Ciudad de México, Mexico, 2008; pp. 80–89. [Google Scholar]
- Genovese, D.B.; Lozano, J.E. The effect of hydrocolloids on the stability and viscosity of cloudy apple juices. Food Hydrocoll. 2001, 15, 1–7. [Google Scholar] [CrossRef]
- Wyatt, N.B.; Gunther, C.M.; Liberatore, M.W. Increasing viscosity in entangled polyelectrolyte solutions by the addition of salt. Polymer (Guildf.) 2011, 52, 2437–2444. [Google Scholar] [CrossRef]
- Cui, S.W. Food Carbohydrates. Chemistry, Physical Properties and Applications; Cui, S.W., Ed.; CRC Press, Taylor & Francis Group, LLC.: Boca Ratón, FL, USA, 2005; ISBN 9780849315749. [Google Scholar]
- Megías-Pérez, R.; Gamboa-Santos, J.; Soria, A.C.; Villamiel, M.; Montilla, A. Survey of quality indicators in commercial dehydrated fruits. Food Chem. 2014, 150, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.B.; Graham, A. Dinitrosalicylic Acid: A reagent for the estimation of sugar in normal and diabetic urine. J. Biol. Chem. 1921, 47, 5–9. [Google Scholar]
- McCleary, B.V. Development of an integrated total dietary fiber method consistent with the codex alimentarius definition. Cereal Foods World 2010, 55, 24–28. [Google Scholar] [CrossRef]
- Soria, A.C.; Corzo-Martínez, M.; Montilla, A.; Riera, E.; Gamboa-Santos, J.; Villamiel, M. Chemical and physicochemical quality parameters in carrots dehydrated by power ultrasound. J. Agric. Food Chem. 2010, 58, 7715–7722. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Babbar, N.; Van Roy, S.; Wijnants, M.; Dejonghe, W.; Caligiani, A.; Sforza, S.; Elst, K. Effect of Extraction Conditions on the Saccharide (Neutral and Acidic) Composition of the Crude Pectic Extract from Various Agro-Industrial Residues. J. Agric. Food Chem. 2016, 64, 268–276. [Google Scholar] [CrossRef]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A.; Sowndhararajan, K. Comparison of Acidic and Enzymatic Pectin Extraction from Passion Fruit Peels and Its Gel Properties. J. Food Process Eng. 2016, 39, 501–511. [Google Scholar] [CrossRef]
- Garna, H.; Mabon, N.; Nott, K.; Wathelet, B.; Paquot, M. Kinetic of the hydrolysis of pectin galacturonic acid chains and quantification by ionic chromatography. Food Chem. 2006, 96, 477–484. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singthong, J.; Cui, S.W.; Ningsanond, S.; Goff, H.D. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydr. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Montilla, A.; Moreno, F.J.; Villamiel, M. Modification of citrus and apple pectin by power ultrasound: Effects of acid and enzymatic treatment. Ultrason. Sonochem. 2017, 38, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, L.-J.; Li, D.; Adhikari, B.; Shi, J. Effect of gum arabic on stability of oil-in-water emulsion stabilized by flaxseed and soybean protein. Carbohydr. Polym. 2011, 86, 343–351. [Google Scholar] [CrossRef]
- Falk, G.; Borlaf, M.; Bendo, T.; Novaes de Oliveira, A.P.; Rodrigues Neto, J.B.; Moreno, R. Colloidal sol-gel synthesis and photocatalytic activity of nanoparticulate Nb2O5 sols. J. Am. Ceram. Soc. 2016, 99, 1968–1973. [Google Scholar] [CrossRef]
Sample Availability: Samples of the pectin are available from the authors. |





| Parameter | SBP-P | SBP-E | SBP-D |
|---|---|---|---|
| °Brix | 5.00 ± 0.21 b | 4.60 ± 0.07 a | 4.40 ± 0.14 a |
| pH | 4.62 ± 0.08 c | 3.51 ± 0.03 a | 3.71 ± 0.06 a,b |
| Aw | 0.88 ± 0.01 b | 0.90 ± 0.02 b | 0.73 ± 0.02 a |
| DW (%) | 91.12 ± 0.16 b | 83.41 ± 0.15 a | 96.53 ± 0.22 c |
| Total fat (g/100 g DW) | 0.84 ± 0.02 a | 1.70 ± 0.04 c | 1.33 ± 0.03 b |
| Protein (g/100 g DW) | 10.42 ± 0.52 c | 8.30 ± 0.30 ab | 8.01 ± 0.31 a |
| Total carbohydrates (g/100 g DW) | 82.64 ± 0.63 c | 70.22 ± 0.32 a | 78.14 ± 0.50 b |
| Reducing carbohydrates (g/100 g DW) | 10.40 ± 0.11 c | 6.73 ± 0.10 b | 4.21 ± 0.08 a |
| TDF (g/100 g DW) | 75.20 ± 0.24 b | 64.52 ± 0.07 a | 76.84 ± 0.32 b,c |
| IDF (g/100 g DW) | 47.58 ± 0.16 b | 34.51 ± 0.09 a | 51.32 ± 0.18 b,c |
| SDF (g/100 g DW) | 26.63 ± 0.47 a | 30.04 ± 0.50 b,c | 29.78 ± 0.34 b |
| Ash (g/100 g DW) | 1.86 a,b | 1.87 a,b | 1.81 a |
| Na+ (mg/100 g DW) | 17.66 a,b | 26.39 c | 16.16 a |
| Mg+2 (mg/100 g DW) | 244.97 c | 217.16 a,b | 214.30 a |
| P+3 (mg/100 g DW) | 27.51 a,b | 24.20 a | 23.55 a |
| K+ (mg/100 g DW) | 184.72 b | 189.84 b,c | 174.68 a |
| Ca+2 (mg/100 g DW) | 1327.90 a,b | 1332.21 b,c | 1317.26 a |
| Fe+3 (mg/100 g DW) | 54.63 a | 84.95 c | 62.45 a,b |
| Total phenols (mg GAE/100 g DW) | 0.38 ± 0.05 c | 0.29 ± 0.02 b | 0.17 ± 0.01 a |
| Antioxidant capacity (mM de Trolox/100 g DW) | 2.34 ± 0.14 b,c | 2.23 ± 0.09 b | 1.16 ± 0.10 a |
| Pectin | Extraction Method | Yield | Xylose | Arabinose | Rhamnose | Galactose | Galacturonic Acid | Mannose | Glucose | Protein |
|---|---|---|---|---|---|---|---|---|---|---|
| P-SBP-P | AM | 13.60 | 33.35 ± 1.11 f | 3.60 ± 0.08 e,f | 9.81 ± 0.31 e | 14.50 ± 0.50 d | 23.52 ± 0.11 a | 6.14 ± 0.18 e | 2.01 ± 0.02 b | 4.3 ± 0.22 e,f |
| EM | 3.91 | 6.36 ± 0.19 c | 0.10 ± 0.00 a | 3.20 ± 0.06 a | 8.26 ± 0.27 a,b | 65.51 ± 0.30 e | 4.04 ± 0.03 c | 5.22 ± 0.09 d | 1.6 ± 0.06 a | |
| P-SBP-E | AM | 18.94 | 25.48 ± 0.69 d,e | 3.22 ± 0.06 e | 4.94 ± 0.13 c,d | 7.60 ± 0.28 a | 42.74 ± 0.23 b | 2.39 ± 0.04 a | 3.30 ± 0.03 c | 3.4 ± 0.14 d |
| EM | 13.40 | 4.53 ± 0.14 a | 0.70 ± 0.01 b | 3.50 ± 0.11 a,b | 9.68 ± 0.33 c | 66.98 ± 0.80 ef | 5.05 ± 0.15 d | 0.76 ± 0.01 a | 2.0 ± 0.08 b | |
| P-SBP-D | AM | 16.72 | 21.53 ± 0.85 d | 2.82 ± 0.06 d | 4.74 ± 0.15 c | 8.88 ± 0.24 b | 48.92 ± 0.43 d | 4.75 ± 0.17 d | 0.62 ± 0.01 a | 4.1 ± 0.20 e |
| EM | 7.50 | 5.22 ± 0.16 a,b | 0.79 ± 0.02 b,c | 16.79 ± 0.69 f | 8.68 ± 0.26 b | 44.80 ± 0.37 b,c | 3.06 ± 0.09 b | 12.57 ± 0.30 e | 2.8 ± 0.1 c |
| Pectin | Extraction Method | DM | * Mw |
|---|---|---|---|
| P-SBP-P | AM | 49.29 a | 306 ± 7 a |
| EM | 47.08 a | 311 ± 9 a | |
| P-SBP-E | AM | 50.14 a | 303 ± 7 a |
| EM | 48.36 a | 322 ± 10 a | |
| P-SBP-D | AM | 48.39 a | 315 ± 9 a |
| EM | 45.21 a | 319 ± 10 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, M.T.; Villamiel, M.; Moreno, R.; Moreno, F.J. Structural and Rheological Properties of Pectins Extracted from Industrial Sugar Beet By-Products. Molecules 2019, 24, 392. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24030392
Pacheco MT, Villamiel M, Moreno R, Moreno FJ. Structural and Rheological Properties of Pectins Extracted from Industrial Sugar Beet By-Products. Molecules. 2019; 24(3):392. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24030392
Chicago/Turabian StylePacheco, M. Teresa, Mar Villamiel, Rodrigo Moreno, and F. Javier Moreno. 2019. "Structural and Rheological Properties of Pectins Extracted from Industrial Sugar Beet By-Products" Molecules 24, no. 3: 392. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24030392