Engineering of Antimicrobial Surfaces by Using Temporin Analogs to Tune the Biocidal/antiadhesive Effect
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of the Free Temporins in Solution
2.2. Surface Characterization
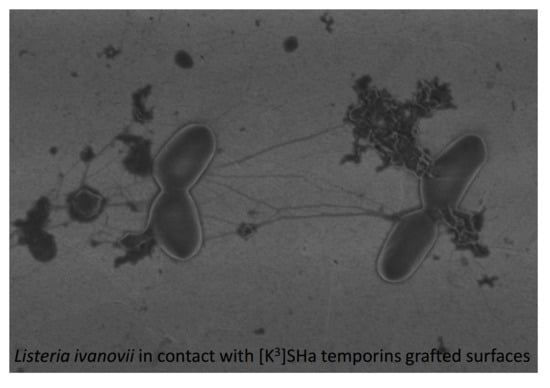
2.3. Antibacterial Activity of Grafted Temporins
3. Materials and Methods
3.1. Reagents
3.2. Peptide Synthesis and Purification
3.3. Surface Preparation
3.4. Polarized Modulated Reflection Absorption Infrared Spectroscopy (PM-RAIRS)
3.5. X-Ray Photoelectron Spectroscopy (XPS)
3.6. Scanning Electron Microscopy with Field Emission Gun (SEM-FEG)
3.7. Water Contact Angle Measurements
3.8. Bacterial Strain and Culture Conditions
3.9. Deposition of Bacteria on Samples
3.10. Evaluation of Bacteria Adhesion by Infrared Spectroscopy
3.11. Observation of Bacterial Morphology by Microscopy
3.12. Bacteria Growth Capacity
3.13. Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) Determination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zobell, C.E. The Effect of Solid Surfaces upon Bacterial Activity. J. Bacteriol. 1943, 46, 39–56. [Google Scholar] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; De Beer, D.; Caldwell, D.; Korber, D.; James, G. Biofilms, the customized microniche. J. Bacteriol. 1994, 176, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Drenkard, E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microb. Infect. 2003, 5, 1213–1219. [Google Scholar] [CrossRef]
- Bridier, A.; Le Coq, D.; Dubois-Brissonnet, F.; Thomas, V.; Aymerich, S.; Briandet, R. The spatial architecture of Bacillus subtilis biofilms deciphered using a surface-associated model and in situ imaging. PLoS ONE 2011, 6, e16177. [Google Scholar] [CrossRef] [PubMed]
- Beech, I.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Caro, A.; Humblot, V.; Méthivier, C.; Minier, M.; Barbes, L.; Li, J.; Salmain, M.; Pradier, C.M. Bioengineering of Stainless Steel Surface by Covalent Immobilisation of Enzymes. Physical Characterisation and Interfacial Enzymatic Activity. J. Colloid Interface Sci. 2010, 349, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Héquet, A.; Humblot, V.; Berjeaud, J.M.; Pradier, C.M. Optimized Grafting of Antimicrobial Peptides on Stainless Steel Surface: Biofilm Resistance Tests. Colloids Surf. B Biointerfaces 2011, 84, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Humblot, V.; Yala, J.F.; Thebault, P.; Boukerma, K.; Hequet, A.; Berjeaud, J.M.; Pradier, C.M. The antibacterial activity of Magainin I immobilized onto mixed thiols Self-Assembled Monolayers. Biomaterials 2009, 30, 3503–3512. [Google Scholar] [CrossRef] [PubMed]
- Hanna, H.; Benjamin, R.; Chatzinikolaou, I.; Alakech, B.; Richardson, D.; Mansfield, P.; Dvorak, T.; Munsell, M.F.; Darouiche, R.; Kantarjian, H.; et al. Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients: A prospective randomized clinical trial. J. Clin. Oncol. 2004, 22, 3163–3171. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.M. Permanently microbicidal materials coatings. J. Mater. Chem. 2007, 17, 2479–2482. [Google Scholar] [CrossRef]
- Tiller, J.C.; Liao, C.J.; Lewis, K.; Klibanov, A.M. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Dowling, D.P.; Donnelly, K.; McConnell, M.L.; Eloy, R.; Arnaud, M.N. Deposition of anti-bacterial silver coatings on polymeric substrates. Thin Solid Films 2001, 398, 602–606. [Google Scholar] [CrossRef]
- Omae, I. Organotin antifouling paints and their alternatives. Appl. Organomet. Chem. 2003, 17, 81–105. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Min-Duk, S.; Hyung-Sik, W.; Ji-Hun, K.; Tsogbadrakh, M.O.; Bong-Jin, L. Antimicrobial Peptides for Therapeutic Applications: A Review. Molecules 2012, 17, 12276–12286. [Google Scholar] [Green Version]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R1–R21. [Google Scholar] [CrossRef] [PubMed]
- Yala, J.F.; Thebault, P.; Hequet, A.; Humblot, V.; Pradier, C.M.; Berjeaud, J.M. Elaboration of antibiofilm materials by chemical grafting of an antimicrobial peptide. Appl. Microbiol. Biotechnol. 2011, 89, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Lombana, A.; Raja, Z.; Casale, S.; Pradier, C.M.; Foulon, T.; Ladram, A.; Humblot, V. Temporin-SHa Peptides Grafted on Gold Surfaces Display Antibacterial Activity. J. Pept. Sci. 2014, 20, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, F.; Oury, B.; Blasco, T.; Sereno, D.; Bolbach, G.; Nicolas, P.; Hani, K.; Amiche, M.; Ladram, A. Isolation, characterization and molecular cloning of new temporins from the skin of the North African ranid Pelophylax saharica. Peptides 2008, 29, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, F.; Galanth, C.; Amiche, M.; Saito, K.; Piesse, C.; Zargarian, L.; Hani, K.; Nicolas, P.; Lequin, O.; Ladram, A. Solution structure and model membrane interactions of temporins-SH, antimicrobial peptides from amphibian skin. A NMR spectroscopy and differential scanning calorimetry study. Biochemistry 2008, 47, 10513–10525. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Al-Ghaferi, N.; Abraham, B.D.; Leprince, J. Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents. Methods 2007, 42, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Galanth, C.; Abbassi, F.; Lequin, O.; Ayala-Sanmartin, J.; Ladram, A.; Nicolas, P.; Amiche, M. Mechanism of antibacterial action of dermaseptin B2: Interplay between helix-hinge-helix structure and membrane curvature strain. Biochemistry 2009, 48, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Raja, Z.; André, S.; Abbassi, F.; Humblot, V.; Lequin, O.; Bouceba, T.; Correia, I.; Casale, S.; Foulon, T.; Sereno, D.; et al. Insight into the mechanism of action of temporin-SHa, a new broad-spectrum antiparasitic and antibacterial agent. PLoS ONE 2017, 12, e0174024. [Google Scholar] [CrossRef] [PubMed]
- French, G.L. Bactericidal agents in the treatment of MRSA infections--the potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boujday, S.; Bantegnie, A.; Briand, E.; Marnet, P.G.; Salmain, M.; Pradier, C.M. In-depth investigation of protein adsorption on gold surfaces: Correlating the structure and density to the efficiency of the sensing layer. J. Phys. Chem. B 2008, 112, 6708–6715. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Troughton, E.B.; Tao, Y.T.; Evall, J.; Whitesides, G.M.; Nuzzo, R.G. Formation of Monolayer Films by the Spontaneous Assembly of Organic Thiols from Solution onto Gold. J. Am. Chem. Soc. 1989, 111, 321–335. [Google Scholar] [CrossRef]
- Tielens, F.; Costa, D.; Humblot, V.; Pradier, C.M. Characterization of ω-functionalized undecanethiol mixed self-assembled monolayers on Au(111): A combined polarization modulation infrared reflection-absorption spectroscopy/X-ray photoelectron spectroscopy/periodic density functional theory study. J. Phys. Chem. C 2008, 112, 182–190. [Google Scholar] [CrossRef]
- Valotteau, C.; Calers, C.; Casale, S.; Berton, J.; Stevens, C.V.; Babonneau, F.; Pradier, C.M.; Humblot, V.; Baccile, N. Biocidal Properties of a Glycosylated Surface: Sophorolipids on Au(111). ACS Appl. Mater. Interfaces 2015, 7, 18086–18095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briand, E.; Salmain, M.; Compère, C.; Pradier, C.M. Immobilization of Protein A on SAMs for the elaboration of immunosensors. Colloids Surf. B Biointerfaces 2006, 53, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Wirde, M.; Gelius, U.; Nyholm, L. Self-assembled monolayers of cystamine and cysteamine on gold studied by XPS and voltammetry. Langmuir 1999, 15, 6370–6378. [Google Scholar] [CrossRef]
- Minier, M.; Salmain, M.; Yacoubi, N.; Barbes, L.; Méthivier, C.; Zanna, S.; Pradier, C.M. Covalent immobilization of lysosyme on stainless steel. Interface spectroscopic characterization and measurement of enzymatic activity. Langmuir 2005, 21, 5957–5965. [Google Scholar] [CrossRef] [PubMed]
- Méthivier, C.; Humblot, V.; Pradier, C.M. L-Methionine adsorption on Cu(110), binding and geometry of the amino acid as a function of coverage. Surf. Sci. 2015, 632, 88–92. [Google Scholar] [CrossRef]
- Schlusselhuber, M.; Humblot, V.; Casale, S.; Méthivier, C.; Verdon, J.; Leippe, M.; Berjeaud, J.M. Potent Antimicrobial Peptides Against Legionella Pneumophila and its Environmental Host, Acanthamoeba castellanii. Appl. Microbiol. Biotechnol. 2015, 99, 4879–4891. [Google Scholar] [CrossRef] [PubMed]
- Tielens, F.; Humblot, V.; Pradier, C.M.; Calatayud, M.; Illas, F. Stability of Binary SAMs Formed by omega-Acid and Alcohol Functionalized Thiol Mixtures. Langmuir 2009, 25, 9980–9985. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Valotteau, C.; Banat, I.M.; Mitchell, C.A.; Lydon, H.; Babonneau, F.; Pradier, C.M.; Baccile, N.; Humblot, V. Antibacterial properties of sophorolipid-modified gold surfaces against Gram positive and Gram negative pathogens. Colloids Surf. B Biointerfaces 2017, 157, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briand, E.; Salmain, M.; Compère, C.; Pradier, C.M. Anti-rabbit immunoglobulin G detection in complex medium by PM-RAIRS and QCM: Influence of the antibody immobilisation method. Biosens. Bioelectron 2007, 22, 2884–2890. [Google Scholar] [CrossRef] [PubMed]
- Pinese, C.; Jebors, S.; Bilak, E.J.; Garric, X.; Humblot, V.; Calers, C.; Martinez, J.; Mehdi, A.; Subra, G. Simple and specific grafting of antibacterial peptides on silicone catheters. Adv. Healthc. Mat. 2016, 5, 3067–3073. [Google Scholar] [CrossRef] [PubMed]
- Greif, D.; Wesner, D.; Regtmeier, J.; Anselmetti, D. High resolution imaging of surface patterns of single bacterial cells. Ultramicroscopy 2010, 110, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Robichon, D.; Girard, J.C.; Cenatiempo, Y.; Cavellier, J.F. Atomic Force Microscopy Imaging of Driedor Living Bacteria. C. R. Acad. Sci. III 1999, 322, 687–693. [Google Scholar] [CrossRef]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N. Antimicrobial peptides from the skins of North American frogs. Biochim. Biophys. Acta 2009, 1788, 1556–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangoni, M.L. Temporins, anti-infective peptides with expanding properties. Cell. Mol. Life Sci. 2006, 63, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-Sections at 1254 and 1487ev. J. Elec. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
Sample Availability: Samples of temporin analogs are available upon request and quotations from the authors through the IBPS synthesis platform. For further details please contact [email protected]. |






| Temporin | MIC (µM) | MBC (µM) | Sequence | Net Charge | Mw a | GRAVY b |
|---|---|---|---|---|---|---|
| SHa | 6 | 6 | FLSGIVGMLGKLFamide | +2 | 1380.76 | 1.67 |
| [A2,6,9]SHa | >200 | >200 | FASGIAGMAGKLFamide | +2 | 1268.55 | 1.18 |
| [K3]SHa | 1.56 | 3.12 | FLKGIVGMLGKLFamide | +3 | 1421.86 | 1.43 |
| d-[K3]SHa | 1.56 | 3.12 | flkGivGmlGklfamide | +3 | 1421.86 | 1.43 |
| [A2,6,9, K3]SHa | >200 | >200 | FAKGIAGMAGKLFamide | +3 | 1309.64 | 0.94 |
| [K3]SHa-COOH | 64 | 128 | FLKGIVGMLGKLFCOOH | +3 | 1422.86 | 1.43 |
| [A2,6,9, K3]SHa-COOH | 128 | 256 | FAKGIAGMAGKLFCOOH | +3 | 1310.64 | 0.94 |
| C1s | O1s | N1s | S2p | |
|---|---|---|---|---|
| MUAM | 79.2 | 13.1 | 4.3 | 3.4 |
| MUAM-[K3]SHa-COOH | 76.4 | 15.4 | 6.8 | 1.4 |
| MUA | 75.0 | 21.0 | - | 4.0 |
| MUA-[K3]SHa | 72.9 | 17.7 | 6.7 | 2.7 |
| MUA | [K3]SHa | d-[K3]SHa | [A2,6,9, K3]SHa | MUAM | [K3]SHa-COOH | [A2,6,9, K3]SHa-COOH | |
|---|---|---|---|---|---|---|---|
| Molecular weight (Da) | 218.4 | 1421.9 | 1421.9 | 1309.6 | 239.8 | 1422.9 | 1310.6 |
| Net charge | N/A | +3 | +3 | +3 | N/A | +2 | +2 |
| Total thickness (Å) | 10.6 ± 2.0 | 32.0 ± 2.9 | 29.2 ± 4.8 | 27.6 ± 3.5 | 11.4 ± 2.0 | 28.1 ± 2.1 | 26.9 ± 2.0 |
| Component thickness (Å) | 10.6 ± 2.0 | 21.4 ± 2.6 | 18.6 ± 2.7 | 17.0 ± 2.6 | 11.4 ± 2.0 | 16.7 ± 2.4 | 15.5 ± 2.4 |
| Molecular surface density (×1014 molecules/cm²) | 3.2 ± 0.6 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.85 ± 0.1 | 3.1 ± 0.5 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Au | MUA | [K3]SHa | D-[K3]SHa | [A2,6,9, K3]SHa | MUAM | [K3]SHa-COOH | [A2,6,9, K3]SHa-COOH | |
|---|---|---|---|---|---|---|---|---|
| Raw Killing (%) | N/A | 13.5 ± 0.2 | 78.9 ± 0.6 | 86.4 ± 1.7 | 7.6 ± 0.9 | 9.1 ± 5.3 | 78.8 ± 2.8 | 22.1 ± 2.2 |
| WCA (°) | 78 ± 2 | 56 ± 3 | 72 ± 3 | 65 ± 2 | 67 ± 3 | 82 ± 1 | 73 ± 2 | 70 ± 2 |
| Adhesion (%) | 100 | 114 ± 15 | 124 ± 25 | 66 ± 15 | 104 ± 22 | 130 ± 25 | 105 ± 10 | 122 ± 18 |
| Corrected Killing (%) | N/A | 1.4 ± 0.2 | 77.0 ± 0.7 | 92.1 ± 1.0 | 3.8 ± 0.5 | 3.2 ± 1.8 | 78.3 ± 2.6 | 4.9 ± 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oger, P.-C.; Piesse, C.; Ladram, A.; Humblot, V. Engineering of Antimicrobial Surfaces by Using Temporin Analogs to Tune the Biocidal/antiadhesive Effect. Molecules 2019, 24, 814. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24040814
Oger P-C, Piesse C, Ladram A, Humblot V. Engineering of Antimicrobial Surfaces by Using Temporin Analogs to Tune the Biocidal/antiadhesive Effect. Molecules. 2019; 24(4):814. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24040814
Chicago/Turabian StyleOger, Pierre-Carl, Christophe Piesse, Ali Ladram, and Vincent Humblot. 2019. "Engineering of Antimicrobial Surfaces by Using Temporin Analogs to Tune the Biocidal/antiadhesive Effect" Molecules 24, no. 4: 814. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24040814