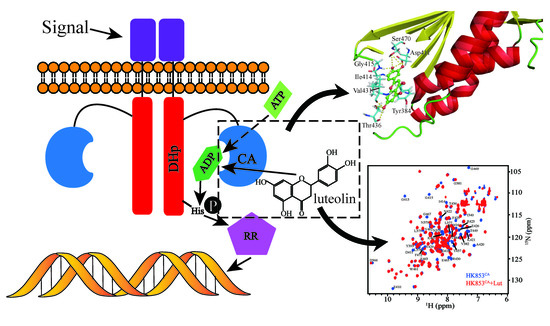
Structural Basis for the Inhibition of the Autophosphorylation Activity of HK853 by Luteolin
Abstract
:1. Introduction
2. Results and Discussions
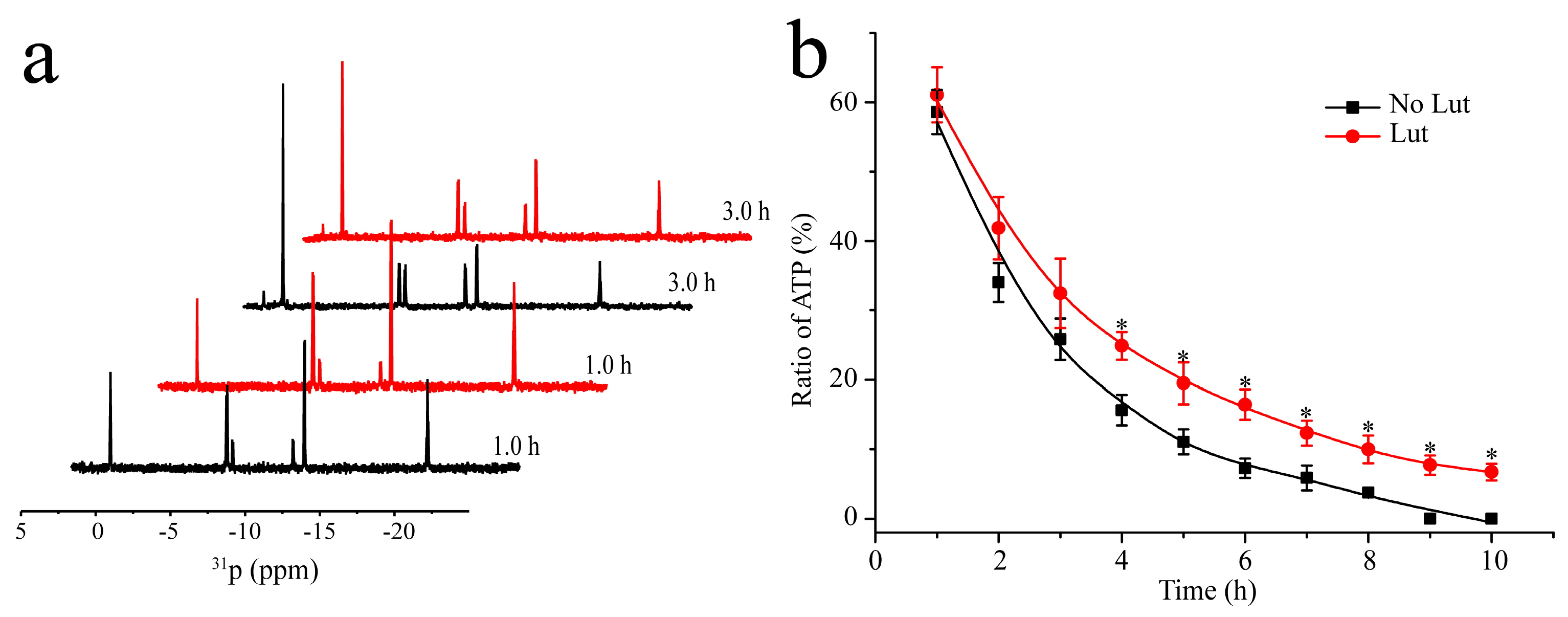
2.1. Inhibition of the Autophosphorylation Activity of HKs by Lut
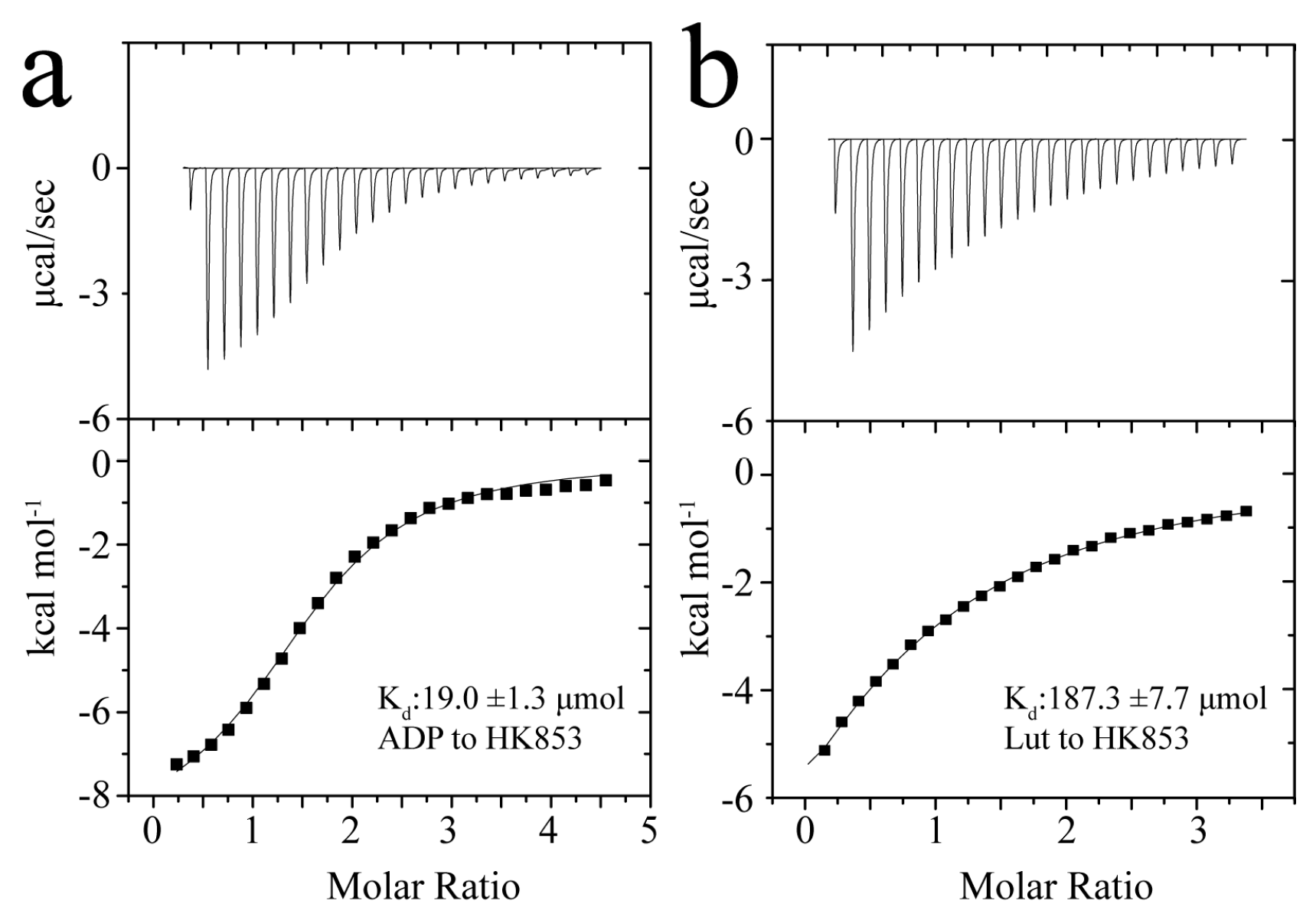
2.2. Determination of the Binding Affinities of Lut/ADP to HK853cp
2.3. Detection of the Binding Site and Conformational Changes
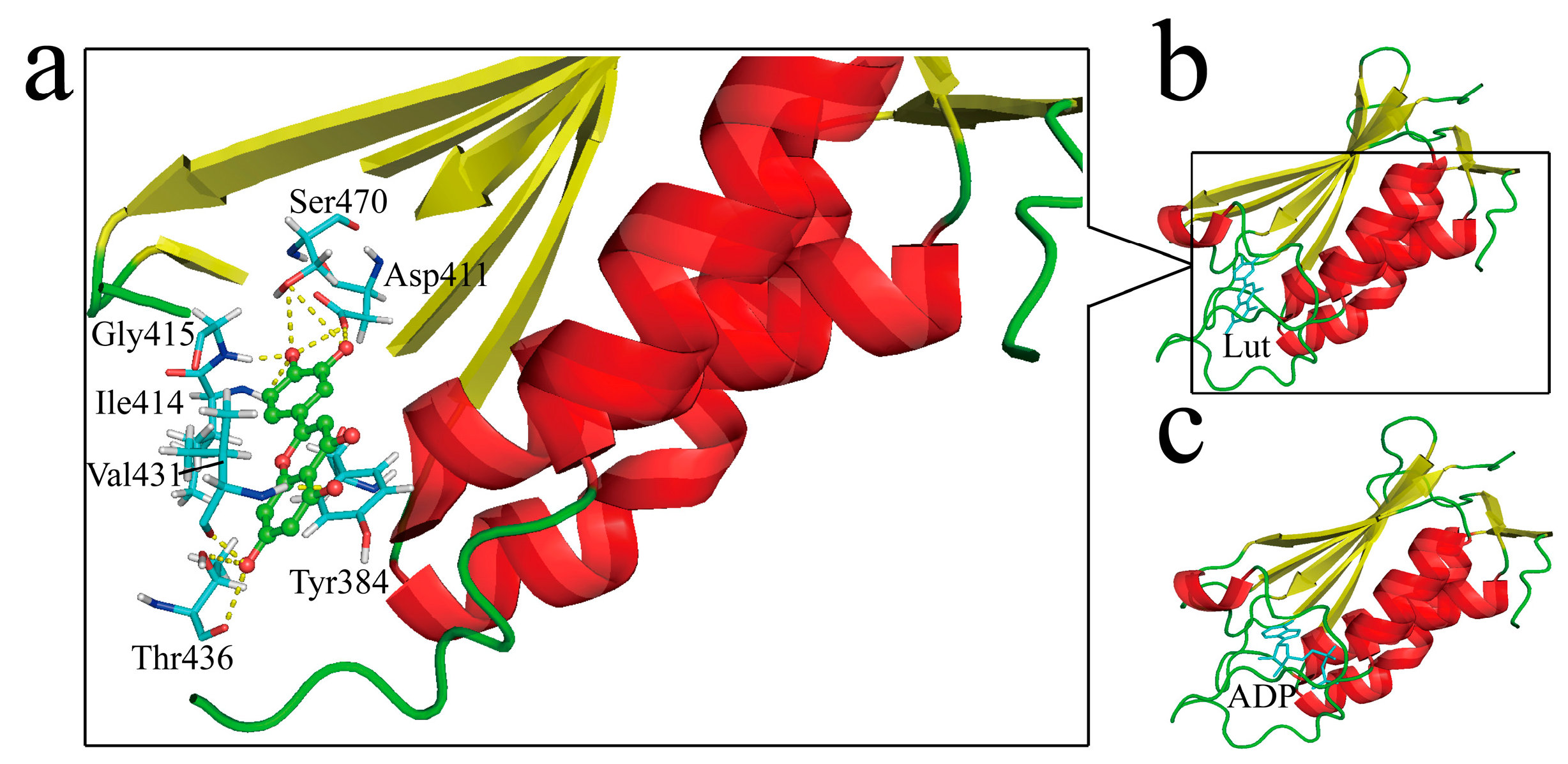
2.4. Structural Insights of Lut Inhibition Suggested by Molecular Docking
2.5. Verification of the Inhibition Mechanism by Other Flavones
3. Materials and Methods
3.1. Reagents
3.2. Protein Preparation and Purification
3.3. ITC Experiments
3.4. NMR Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug. Resist. Updat. 2016, 26, 43–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Landis, R.F.; Li, C.H.; Schnurr, M.; Das, R.; Lee, Y.W.; Yazdani, M.; Liu, Y.; Kozlova, A.; Rotello, V.M. Engineered Polymer Nanoparticles with Unprecedented Antimicrobial Efficacy and Therapeutic Indices against Multidrug-Resistant Bacteria and Biofilms. J. Am. Chem. Soc. 2018, 140, 12137–12143. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Zhu, W.; Hendricks, G.L.; Van Tyne, D.; Steele, A.D.; Keohane, C.E.; Fricke, N.; Conery, A.L.; Shen, S.; Pan, W.; et al. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 2018, 556, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Bem, A.E.; Velikova, N.; Pellicer, M.T.; van Baarlen, P.; Marina, A.; Wells, J.M. Bacterial histidine kinases as novel antibacterial drug targets. ACS Chem. Biol. 2015, 10, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-component signal transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Stock, J.B.; Stock, A.M.; Mottonen, J.M. Signal transduction in bacteria. Nature 1990, 344, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Hoch, J.A. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 2000, 3, 165–170. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mizusaki, H.; Kenney, L.J. A FRET-based DNA biosensor tracks OmpR-dependent acidification of Salmonella during macrophage infection. PLoS Biol. 2015, 13, e1002116. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, F.; Peng, W.; Yan, K.; Zhao, H.; Liu, T.; Cheng, H.; Chang, P.; Yuan, F.; Chen, H.; et al. The CpxA/CpxR Two-Component System Affects Biofilm Formation and Virulence in Actinobacillus pleuropneumoniae. Front. Cell Infect. Microbiol. 2018, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Skerker, J.M.; Perchuk, B.S.; Siryaporn, A.; Lubin, E.A.; Ashenberg, O.; Goulian, M.; Laub, M.T. Rewiring the specificity of two-component signal transduction systems. Cell 2008, 133, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Thomason, P.; Kay, R. Eukaryotic signal transduction via histidine-aspartate phosphorelay. J. Cell Sci. 2000, 113, 3141–3150. [Google Scholar] [CrossRef] [PubMed]
- Wilke, K.E.; Francis, S.; Carlson, E.E. Inactivation of multiple bacterial histidine kinases by targeting the ATP-binding domain. ACS Chem. Biol. 2015, 10, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rose, J.; Huang, S.; Hu, Y.; Wu, Q.; Wang, D.; Li, C.; Liu, M.; Zhou, P.; Jiang, L. A pH-gated conformational switch regulates the phosphatase activity of bifunctional HisKA-family histidine kinases. Nat. Commun. 2017, 8, 2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casino, P.; Rubio, V.; Marina, A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell 2009, 139, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Brannigan, J.A.; Muchová, K.; Barák, I.; Wilkinson, A.J. Phosphorylated aspartate in the structure of a response regulator protein. J. Mol. Biol. 1999, 294, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Casino, P.; Miguel-Romero, L.; Marina, A. Visualizing autophosphorylation in histidine kinases. Nat. Commun. 2014, 5, 3258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podgornaia, A.I.; Casino, P.; Marina, A.; Laub, M.T. Structural basis of a rationally rewired protein-protein interface critical to bacterial signaling. Structure 2013, 21, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Willett, J.W.; Kirby, J.R. Genetic and biochemical dissection of a HisKA domain identifies residues required exclusively for kinase and phosphatase activities. PLoS Genet. 2012, 8, e1003084. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Yan, H.; Cukier, R.I. Conformational transition of response regulator RR468 in a two-component system signal transduction process. J. Phys. Chem. B 2014, 118, 4727–4742. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.K.; Frost, J.W.; Long, S.R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 1986, 233, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Primikyri, A.; Chatziathanasiadou, M.V.; Karali, E.; Kostaras, E.; Mantzaris, M.D.; Hatzimichael, E.; Shin, J.S.; Chi, S.W.; Briasoulis, E.; Kolettas, E.; et al. Direct binding of Bcl-2 family proteins by quercetin triggers its pro-apoptotic activity. ACS Chem. Biol. 2014, 9, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Silva, F.; Canto-Cavalheiro, M.M.; Menna-Barreto, R.F.; Almeida-Amaral, E.E. Effect of Apigenin on Leishmania amazonensis Is Associated with Reactive Oxygen Species Production Followed by Mitochondrial Dysfunction. J. Nat. Prod. 2015, 78, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.B.; Wang, X.; Nunez Lopez, O.; Graham, G.; Tie, H.Y.; Zhou, J.; Radhakrishnan, R.S. Luteolin-Mediated Inhibition of Hepatic Stellate Cell Activation via Suppression of the STAT3 Pathway. Int. J. Mol. Sci. 2018, 19, 1567. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, P.; Xu, T.; Pan, D.; Zhu, H.; Zhai, N.; Zhang, Y.; Li, D. Luteolin Modulates SERCA2a Leading to Attenuation of Myocardial Ischemia/ Reperfusion Injury via Sumoylation at Lysine 585 in Mice. Cell Physiol. Biochem. 2018, 45, 883–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Zhang, H.; Cai, X.; Fang, W.; Chai, D.; Wen, Y.; Chen, H.; Chu, F.; Zhang, Y. Luteolin Promotes Cell Apoptosis by Inducing Autophagy in Hepatocellular Carcinoma. Cell Physiol. Biochem. 2017, 43, 1803–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Qiu, Y.; Luo, Q.; Zhao, L.; Yan, X.; Ding, Q.; Jiang, H.; Yang, H. The Mechanism by Which Luteolin Disrupts the Cytoplasmic Membrane of Methicillin-Resistant Staphylococcus aureus. J. Phys. Chem. B 2018, 122, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ma, L.; Wen, Y.; Wang, H.; Zhang, S. Studies of the in vitro antibacterial activities of several polyphenols against clinical isolates of methicillin-resistant Staphylococcus aureus. Molecules 2014, 19, 12630–12639. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Zhong, H.J.; Yang, G.; Wu, C.; Huang, J.M.; Li, G.; Ma, D.L.; Leung, C.H. Small Molecule Pin1 Inhibitor Blocking NF-κB Signaling in Prostate Cancer Cells. Chem. Asian J. 2018, 13, 275–279. [Google Scholar] [CrossRef]
- Lu, J.J.; Bao, J.L.; Wu, G.S.; Xu, W.S.; Huang, M.Q.; Chen, X.P.; Wang, Y.T. Quinones derived from plant secondary metabolites as anti-cancer agents. Anticancer Agents Med. Chem. 2013, 13, 456–463. [Google Scholar] [CrossRef]
- Leung, K.H.; Liu, L.J.; Lin, S.; Lu, L.; Zhong, H.J.; Susanti, D.; Rao, W.; Wang, M.; Che, W.I.; Chan, D.S.; et al. Discovery of a small-molecule inhibitor of STAT3 by ligand-based pharmacophore screening. Methods 2015, 71, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.J.; Mayville, P.; Muir, T.W.; Novick, R.P. Rational design of a global inhibitor of the virulence response in Staphylococcus aureus, based in part on localization of the site of inhibition to the receptor-histidine kinase, AgrC. Proc. Natl. Acad. Sci. USA 2000, 21, 13330–13335. [Google Scholar] [CrossRef] [PubMed]
- Lolli, G.; Cozza, G.; Mazzorana, M.; Tibaldi, E.; Cesaro, L.; Donella-Deana, A.; Meggio, F.; Venerando, A.; Franchin, C.; Sarno, S.; et al. Inhibition of protein kinase CK2 by flavonoids and tyrphostins. A structural insight. Biochemistry 2012, 51, 6097–6107. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Kosaka, Y.; Mizuguchi, M. Structural Insight into the Interactions between Death-Associated Protein Kinase 1 and Natural Flavonoids. J. Med. Chem. 2015, 58, 7400–7408. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Langworthy, T.A.; Konig, H.; Thomm, M.; Woese, C.R.; Sleytr, U.B.; Stetter, K.O. Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90 °C. Arch. Microbiol. 1986, 144, 324–333. [Google Scholar] [CrossRef]
- Reith, F.; Lengke, M.F.; Falconer, D.; Craw, D.; Southam, G. The geomicrobiology of gold. ISME J. 2007, 1, 567–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santa-Maria, M.C.; Chou, C.J.; Yencho, G.C.; Haigler, C.H.; Thompson, W.F.; Kelly, R.M.; Sosinski, B. Plant cell calcium-rich environment enhances thermostability of recombinantly produced alpha-amylase from the hyperthermophilic bacterium Thermotoga maritime. Biotechnol. Bioeng. 2009, 104, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Rossi, P.; Saio, T.; Kalodimos, C.G. Structural basis for the antifolding activity of a molecular chaperone. Nature 2016, 537, 202–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saio, T.; Guan, X.; Rossi, P.; Economou, A.; Kalodimos, C.G. Structural basis for protein antiaggregation activity of the trigger factor chaperone. Science 2014, 344, 1250494. [Google Scholar] [CrossRef] [PubMed]
- Botos, I.; Majdalani, N.; Mayclin, S.J.; McCarthy, J.G.; Lundquist, K.; Wojtowicz, D.; Barnard, T.J.; Gumbart, J.C.; Buchanan, S.K. Structural and Functional Characterization of the LPS Transporter LptDE from Gram-Negative Pathogens. Structure 2016, 24, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Khanra, N.; Rossi, P.; Economou, A.; Kalodimos, C.G. Recognition and targeting mechanisms by chaperones in flagellum assembly and operation. Proc. Natl. Acad. Sci. USA 2016, 113, 9798–9803. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, B.; Kelly, B.; McMillan, B.J.; Seegar, T.C.M.; Dror, R.O.; Kruse, A.C.; Blacklow, S.C. Crystal Structure of a Full-Length Human Tetraspanin Reveals a Cholesterol-Binding Pocket. Cell 2016, 167, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sevillano, N.; La Greca, F.; Deis, L.; Liu, Y.C.; Deller, M.C.; Mathews, I.I.; Matsui, T.; Cane, D.E.; Craik, C.S.; et al. Structure–Function Analysis of the Extended Conformation of a Polyketide Synthase Module. J. Am. Chem. Soc. 2018, 140, 6518–6521. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Rossi, P.; Kalodimos, C.G. Atomic view of the energy landscape in the allosteric regulation of Abl kinase. Nat Struct Mol Biol. 2017, 24, 893–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casino, P.; Rubio, V.; Marina, A. The mechanism of signal transduction by two-component systems. Curr. Opin. Struct. Biol. 2010, 20, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Marina, A.; Waldburger, C.D.; Hendrickson, W.A. Structure of the entire cytoplasmic portion of a sensor histidine-kinase protein. EMBO J. 2005, 24, 4247–4759. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Lut, ATP, ADP and AMP are available from the authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Huang, L.; Ji, S.; Hou, S.; Luo, L.; Li, C.; Liu, M.; Liu, Y.; Jiang, L. Structural Basis for the Inhibition of the Autophosphorylation Activity of HK853 by Luteolin. Molecules 2019, 24, 933. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050933
Zhou Y, Huang L, Ji S, Hou S, Luo L, Li C, Liu M, Liu Y, Jiang L. Structural Basis for the Inhibition of the Autophosphorylation Activity of HK853 by Luteolin. Molecules. 2019; 24(5):933. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050933
Chicago/Turabian StyleZhou, Yuan, Liqun Huang, Shixia Ji, Shi Hou, Liang Luo, Conggang Li, Maili Liu, Yixiang Liu, and Ling Jiang. 2019. "Structural Basis for the Inhibition of the Autophosphorylation Activity of HK853 by Luteolin" Molecules 24, no. 5: 933. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050933