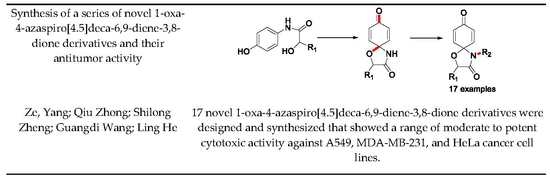
Synthesis and Antitumor Activity of a Series of Novel 1-Oxa-4-azaspiro[4,5]deca-6,9-diene-3,8-dione Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Route to Novel 1-Oxa-4-azaspiro[4.5]deca-6,9-diene-3,8-diones
2.2. Optimization for the Formation of 1-Oxa-4-azaspiro[4.5]deca-6,9-diene-3,8-dione from 2-Hydroxy-N-(4-hydroxyphenyl)acetamide
2.3. Antitumor Activity of 1-Oxa-4-azaspiro[4.5]deca-6,9-diene-3,8-diones
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Synthesis of 1-Oxa-4-azaspiro[4.5]deca-6,9-diene-3,8-diones
3.2.1. Synthesis of Compounds 10a–10b
3.2.2. Synthesis of Compounds 11a–11k
3.2.3. Synthesis of Compounds 12a–12d
3.3. Cell Culture and Antiproliferative Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Zhang, X.; Zeng, R.; Zhang, Y.; Dai, Q.S.; Leng, H.J.; Gou, X.J.; Li, J.L. Recent Advances in the Synthesis of Spiroheterocycles via N-Heterocyclic Carbene Organocatalysis. Molecules 2017, 22, 1882. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, R.S.C.; Feng, P.C. Some pharmacological activities of crotonosine and pronuciferine. J. Pharm. Pharmacol. 1967, 19, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Sun, W.; Wang, J.; Lin, S.; Li, X.N.; Zhu, H.; Luo, Z.; Xue, Y.; Hu, Z.; Zhang, Y. A New Breviane Spiroditerpenoid from the Marine-Derived Fungus Penicillium sp. TJ403-1. Mar. Drugs 2018, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.H.; Chen, K.Q.; Zhang, Y.; Kong, L.M.; Li, Y.; Ye, S. Enantioselective N-Heterocyclic Carbene-Catalyzed Synthesis of Spirocyclic Oxindole-benzofuroazepinones. J. Org. Chem. 2018, 83, 15225–15235. [Google Scholar] [CrossRef] [PubMed]
- Gicquel, M.; Gomez, C.; Garcia Alvarez, M.C.; Pamlard, O.; Guérineau, V.; Jacquet, E.; Bignon, J.; Voituriez, A.; Marinetti, A. Inhibition of p53-Murine Double Minute 2 (MDM2) Interactions with 3,3′-Spirocyclopentene Oxindole Derivatives. J. Med. Chem. 2018, 61, 9386–9392. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Kim, H.N.; Shin, K.D.; Yoon, Y.J.; Kim, S.J.; Han, D.C.; Kwon, B.M. Cryptotanshinone Inhibits Constitutive Signal Transducer and Activator of Transcription 3 Function through Blocking the Dimerization in DU145 Prostate Cancer Cells. Cancer Res. 2009, 69, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Silvers, M.A.; Deja, S.; Singh, N.; Egnatchik, R.A.; Sudderth, J.; Luo, X.; Beg, M.S.; Burgess, S.C.; DeBerardinis, R.J.; Boothman, D.A.; et al. The NQO1 bioactivatable drug, β-Lapachone, alters the redox state of NQO1+ pancreatic cancer cells, causing perturbation in central carbon metabolism. J. Biol. Chem. 2017, 292, 18203–18216. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Cho, J.Y.; Yoon, S.H.; Jang, I.J.; Yu, K.S. Pharmacokinetics and tolerability of MB12066, a beta-lapachone derivative targeting NAD(P)H: Quinone oxidoreductase 1: Two independent, double-blind, placebo-controlled, combined single and multiple ascending dose first-in-human clinical trials. Drug Des. Dev. Ther. 2017, 11, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wang, P.; Zhong, Q.; Deng, Y.; Xie, J.; Liu, F.; Xiao, F.; Zheng, S.; Chen, Y.; Wang, G.; et al. Copper salt-catalyzed formation of a novel series of triazole–spirodienone conjugates with potent anticancer activity. RSC Adv. 2017, 7, 9412–9416. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, F.; Zhong, Q.; Zheng, S.L.; Chen, Y.; Wang, G.D.; He, L. Chemoselective synthesis and cytotoxic activity of a series of novel benzo[1,4]oxazin-3-onederivatives. Chin. Chem. Lett. 2017, 28, 1243–1247. [Google Scholar] [CrossRef]
- Luo, R.; Guo, S.; Zheng, S.; Wang, G.; Bao, X.; He, L. Synthesis and Antitumor Activity of N-Triazol-5-yl-oxazolidin-4-one Derivatives. Chin. J. Org. Chem. 2017, 37, 2435–2441. [Google Scholar] [CrossRef]
- Deng, Y.X.; Xie, J.P.; Zhang, W.W.; Yin, P.; Yu, J.; He, L. Oxidative Amidation of Aromatic Ethers Catalyzed by Rhodium Acetate. Chem. Eur. J. 2012, 18, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, D.J.; Basak, A. N-Methoxy-N-acylnitrenium Ions: Application to the Formal Synthesis of (−)-TAN1251A. Org. Lett. 2001, 3, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Yakura, T.; Haruta, J.; Kita, Y. Hypervalent iodine oxidation of p-alkoxyphenols and related compounds: A general route to p-benzoquinone monoacetals and spiro lactones. J. Org. Chem. 1987, 52, 3927–3930. [Google Scholar] [CrossRef]
- Stepakov, A.V.; Ledovskaya, M.S.; Boitsov, V.M.; Molchanov, A.P.; Kostikov, R.R.; Gurzhiy, V.V.; Starova, G.L. Synthesis of isoxazolopyrroloisoquinolines by intramolecular cyclizations of 5-(2-arylethyl)-6- hydroxytetrahydro-4H-pyrrolo[3,4-d]isoxazol-4-ones. Tetrahedron Lett. 2012, 53, 5414–5417. [Google Scholar] [CrossRef]
- Riedl, C.A.; Flocke, L.S.; Hejl, M.; Roller, A.; Klose, M.H.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. Introducing the 4-Phenyl-1,2,3-Triazole Moiety as a Versatile Scaffold for the Development of Cytotoxic Ruthenium(II) and Osmium(II) Arene Cyclometalates. Inorg. Chem. 2017, 56, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, K. Efficient One-Pot Synthesis of 1,2,3-Triazoles from Benzyl and Alkyl Halides. Synlett 2005, 2005, 0943–0946. [Google Scholar] [CrossRef]
- Parish, E.J.; Qin, H. Tetrakis(acetonitrile)copper(I) Perchlorate. In Encyclopedia of Reagents for Organic Synthesis; Paquette, L.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Canesi, S.; Bouchu, D.; Ciufolini, M.A. Nitrogenous Educts through Oxidative Amidation of Phenols: The Bimolecular Reaction. Org. Lett. 2005, 7, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, J.; Du, F.; Zeng, X.; Li, L.; Zhang, H. Oxidative Carbon-Carbon Bond Formation in the Synthesis of Bioactive Spiro β-Lactams. Org. Lett. 2009, 11, 2820–2823. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, D.J.; Burge, M.S.; Zhang, W.; Ortiz, J.A. π-Face selective azaspirocyclization of ω-(methoxyphenyl)-N-methoxyalkylamides. Tetrahedron Lett. 2003, 44, 2587–2591. [Google Scholar] [CrossRef]
- Wardrop, D.J.; Landrie, C.L.; Ortiz, J.A. Total Synthesis of the Coccinellid Alkaloid (±)-Adalinine Utilizing a Nitrenium Ion Cyclization. Synlett 2003, 2003, 1352–1354. [Google Scholar] [CrossRef]
- Tanaka, K.; Mori, Y.; Narasaka, K. Synthesis of Spiro[indoline-3,2′-pyrrolidine] Derivatives from β-3-Indolyl Ketone Oximes. Chem. Lett. 2004, 33, 26–27. [Google Scholar] [CrossRef]
- Narasaka, K. Metal-assisted amination with oxime derivatives. Pure Appl. Chem. 2002, 74, 143–149. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Entry | Oxidizing Agent (2 eq) | Metal Catalyst (0.05 eq) | Yield (%) |
|---|---|---|---|
| 1 | PhI(OAc)2 | Cu[(CH3CN)4ClO4] | 72 |
| 2 | PhI(OAc)2 | Rh2(OAc)4 | 75 |
| 3 | PhI(OAc)2 | Mn(OAc)2 | trace |
| 4 | PhI(OAc)2 | FeCl2 | trace |
| 5 | PhI(OAc)2 | ZnCl2 | trace |
| 6 | PIFA | Cu[(CH3CN)4ClO4] | 68 |
| 7 | PhI(OAc)2 | No | trace |
| Entry | Base (1.5 eq) | Temperature (°C) | Yield (%) |
|---|---|---|---|
| 1 | K2CO3 | 25 | trace |
| 2 | NaH | 0 | 50 |
| 3 | DBU | 0-25 | 67 |
| 4 | DABCO | 80 | trace |
| 5 | Et3N | 25 | trace |
| 6 | No | 25 | no reaction |
| Compound | IC50 (μM) | ||
|---|---|---|---|
| A549 | MDA-BM-231 | HeLa | |
| 10a | 0.23 ± 0.15 | 0.28 ± 0.07 | 0.26 ± 0.12 |
| 10b | 0.52 ± 0.27 | 0.18 ± 0.08 | 1.34 ± 0.28 |
| 11a | 0.34 ± 0.09 | 0.23 ± 0.06 | 0.47 ± 0.13 |
| 11b | 0.18 ± 0.01 | 0.12 ± 0.06 | 0.27 ± 0.15 |
| 11c | 0.37 ± 0.11 | 0.13 ± 0.07 | 0.22 ± 0.05 |
| 11d | 0.72 ± 0.09 | 0.09 ± 0.02 | 0.20 ± 0.05 |
| 11e | 0.29 ± 0.10 | 0.15 ± 0.04 | 0.31 ± 0.16 |
| 11f | 0.24 ± 0.08 | 0.11 ± 0.05 | 0.19 ± 0.12 |
| 11g | 0.26 ± 0.14 | 0.10 ± 0.03 | 0.20 ± 0.07 |
| 11h | 0.19 ± 0.03 | 0.08 ± 0.02 | 0.15 ± 0.02 |
| 11i | 0.81 ± 0.13 | 0.21 ± 0.11 | 0.27 ± 0.14 |
| 11j | 0.39 ± 0.14 | 0.17 ± 0.06 | 0.60 ± 0.12 |
| 11k | 0.26 ± 0.08 | 0.08 ± 0.004 | 0.14 ± 0.07 |
| 12a | >10 | 4.75 ± 0.90 | >10 |
| 12b | 0.76 ± 0.10 | 0.27 ± 0.04 | 0.41 ± 0.04 |
| 12c | 0.90 ± 0.03 | 0.31 ± 0.01 | 0.14 ± 0.1 |
| 12d | 5.31 ± 1.01 | 0.31 ± 0.08 | 1.65 ± 0.46 |
| bendamustine | - | 13.28 ± 0.53 | >20 |
| vorinostat | - | 3.62 ± 0.18 | 4.52 ± 0.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhong, Q.; Zheng, S.; Wang, G.; He, L. Synthesis and Antitumor Activity of a Series of Novel 1-Oxa-4-azaspiro[4,5]deca-6,9-diene-3,8-dione Derivatives. Molecules 2019, 24, 936. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050936
Yang Z, Zhong Q, Zheng S, Wang G, He L. Synthesis and Antitumor Activity of a Series of Novel 1-Oxa-4-azaspiro[4,5]deca-6,9-diene-3,8-dione Derivatives. Molecules. 2019; 24(5):936. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050936
Chicago/Turabian StyleYang, Ze, Qiu Zhong, Shilong Zheng, Guangdi Wang, and Ling He. 2019. "Synthesis and Antitumor Activity of a Series of Novel 1-Oxa-4-azaspiro[4,5]deca-6,9-diene-3,8-dione Derivatives" Molecules 24, no. 5: 936. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050936