3.2. Synthesis
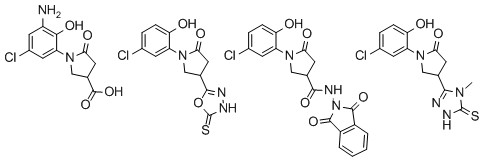
1-(5-Chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic acid (
1) [
23]: A mixture of itaconic acid (97.57 g, 0.75 mol) dissolved in water (200 mL) and 2-amino-4-chlorophenol (71.79 g, 0.5 mol) was heated at reflux for 24 h. Afterwards, it was cooled down to room temperature and diluted with aqueous 10% NaOH solution (300 mL). The solution was heated to the reflux temperature and cooled down to 20 °C. Then it was filtered and filtrate was acidified with HCl to pH 2. Precipitate was filtered off, washed with water, and recrystallized by dissolving in aqueous 5% NaOH solution (100 mL) and pouring into 10% HCl solution to pH 1. Yield 66%; beige solid; m.p. 176−177 °C; IR (KBr) ν
max (cm
−1): 3174 (OH), 1740, 1637 (C=O);
1H-NMR and
13C-NMR were found to be identical with the ones described in [
23]; HRMS (ESI):
m/
z calcd for C
11H
11ClNO
4 256.0376 [M + H]
+, found 256.0374.
1-(3,5-Dichloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic acid (2): A mixture of 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic acid (1) (0.26 g, 1 mmol), 6 M hydrochloric acid (10 mL), and acetic acid (4 mL) was stirred at room temperature for 2 h. Afterwards, 30% hydrogen peroxide (6 mL) was slowly added and stirring was continued for 12 h. Water (10 mL) was added, precipitate formed was filtered off, and recrystallized from ethanol. Yield 86%; beige solid; m.p. 189–190 °C; IR (KBr) νmax (cm−1): 3375 (OH), 1710, 1667 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 2.59–2.71 (m, 2H, COCH2), 3.43 (qui, J = 8.0 Hz, 1H, CH), 3.84 (d, J = 8.4 Hz, 2H, NCH2), 7.27 (s, 1H, Ar-H), 7.48 (s, 1H, Ar-H), 10.03 (s, 1H, OH), 12.72 (br s, 1H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 33.5 (CH), 36.3 (CH2), 50.8 (NCH2), 122.4, 122.5, 126.8, 128.1, 128.3, 148.5 (Ar-C), 172.8, 174.0 (C=O); HRMS (ESI): m/z calcd for C11H10Cl2NO4 289.9987 [M + H]+, found 289.9985.
1-(5-Chloro-2-hydroxy-3-nitrophenyl)-5-oxopyrrolidine-3-carboxylic acid (3): A mixture of 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic acid (1) (0.26 g, 1 mmol) and 30% HNO3 (10 mL) was stirred at room temperature until acid 1 completely dissolved. Precipitate formed after 10 min was filtered off, washed with water, and recrystallized from propan-2-ol. Yield 98%; yellow solid; m.p. 188–189 °C (propan-2-ol); IR (KBr) νmax (cm−1): 3267 (OH), 1705, 1668 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 2.61–2.72 (m, 2H, COCH2), 3.44 (qui, J = 8.0 Hz, 1H, CH), 3.84–3.92 (m, 2H, NCH2), 7.68 (s, 1H, Ar-H), 7.97 (s, 1H, Ar-H), 10.96 (br s, 1H, OH), 12.76 (br s, 1H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 33.4 (CH), 36.3 (CH), 50.7 (NCH2), 122.0, 123.4, 130.2, 133.5, 138.9, 147.0 (Ar-C), 172.9, 173.9 (C=O); HRMS (ESI): m/z calcd for C11H10ClN2O6 301.0227 [M + H]+, found 301.0222.
1-(5-Chloro-2-hydroxy-3-isopropylphenyl)-5-oxopyrrolidine-3-carboxylic acid (4): A mixture of 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic acid (1) (0.26 g, 1 mmol), 80% H2SO4, and propan-2-ol (10 mL) was stirred at 80 °C for 4 h. Afterwards, cold water (10 mL) was added. Precipitate was filtered off, washed with water, and recrystallized from propan-2-ol. Yield 90%; white solid; m.p. 158–159 °C; IR (KBr) νmax (cm−1): 3140 (OH), 1718, 1664 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 1.15, 1.17 (2s, 6H, 2CH3), 2.66 (d, J = 8.4 Hz, 2H, COCH2), 3.25 (qui, J = 6.8 Hz, 1H, CH), 3.44 (qui, J = 8.4 Hz, 1H, CH), 3.79–3.89 (m, 2H, NCH2), 7.06 (s, 1H, Ar-H), 7.11 (s, 1H, Ar-H), 8.98 (br s, 2H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 22.3 (CH3), 26.7 (CH), 33.8 (CH), 36.3 (CH2), 51.1 (NCH2), 122.6, 124.4, 124.9, 126.9, 138.8, 148.9 (Ar-C), 173.1, 174.2 (C=O); HRMS (ESI): m/z calcd for C14H17ClNO4 298.0846 [M + H]+, found 298.0841.
1-(5-Chloro-2-hydroxy-3-nitrosophenyl)-5-oxopyrrolidine-3-carboxylic acid (5): To 1-(5-chloro-2-hydroxy-3-nitrophenyl)-5-oxopyrrolidine-3-carboxylic acid (3) (0.30 g, 1 mmol) dissolved in propan-2-ol (10 mL), Raney Ni (1 g) was added and a reaction mixture was stirred at room temperature for 3 h. Raney Ni was separated by filtration, the precipitate formed in a filtrate was filtered off, washed with water, and recrystallized from propan-2-ol. Yield 85%; khaki solid; m.p. 210–211 °C; IR (KBr) νmax (cm−1): 3197 (OH), 1687(C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 2.66 (d, J = 8.8 Hz, 2H, COCH2), 3.45 (qui, J = 8.8 Hz, 1H, CH), 3.84–3.91 (m, 2H, NCH2), 7.66 (s, 1H, Ar-H), 7.95 (s, 1H, Ar-H), 11.74 (br s, 2H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 33.3 (CH), 36.3 (CH2), 50.6 (NCH2), 121.7, 123.3, 130.2, 133.4, 138.9, 147.2 (Ar-C), 172.9, 173.9 (C=O); HRMS (ESI): m/z calcd for C11H10ClN2O5 285.0278 [M + H]+, found 285.0472.
1-(3-Amino-5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic acid (6): To 1-(5-chloro-2-hydroxy-3-nitrophenyl)-5-oxopyrrolidine-3-carboxylic acid (3) (1.20 g, 4 mmol) dissolved in propan-2-ol (15 mL), Raney Ni (8 g) was added and a reaction mixture was heated at reflux for 6 h. Raney Ni was separated by filtration, the filtrate was diluted with diethyl ether (15 mL), precipitate formed was filtered off and recrystallized from propan-2-ol. Yield 51%; beige solid; m.p. 159–160 °C; IR (KBr) νmax (cm−1): 3268 (NH2), 3065 (OH), 1705 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 2.23–2.30 (m, 1H, COCH2), 2.68–2.74 (m, 1H, COCH2), 2.81–2.88 (m, 1H, CH), 3.61–3.68 (m, 2H, NCH2), 4.95 (s, 2H, NH2), 6.29 (s, 1H, Ar-H), 6.55 (s, 1H, Ar-H), 8.00 (br s, 2H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 25.5 (CH), 36.3 (CH2), 52.3 (NCH2), 111.8, 114.1, 121.4, 125.0, 139.6, 139.8 (Ar-C), 173.6, 177.4 (C=O); HRMS (ESI): m/z calcd for C11H12ClN2O4 271.0485 [M + H]+, found 271.0482.
1-(5-Chloro-2-oxo-2,3-dihydrobenzo[d]oxazol-7-yl)-5-oxopyrrolidine-3-carboxylic acid (7): To 1-(3-amino-5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic acid (6) (0.16 g, 0.6 mmol) dissolved in propan-2-ol (20 mL), CO(NH2)2 (72 mg, 1.2 mmol) was added and a reaction mixture was heated at reflux for 4 h. Precipitate was filtered off, washed with water, and recrystallized from propan-2-ol. Yield 69%; brown solid; m.p. 153–154 °C; IR (KBr) νmax (cm−1): 3131 (OH), 3050 (NH), 1765, 1730, 1693 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 2.64–2.80 (m, 2H, COCH2), 3.35–3.46 (m, 1H, CH), 4.03–4.15 (m, 2H, NCH2), 7.02 (s, 1H, Ar-H), 7.42 (s, 1H, Ar-H), 12.00 (br s, 1H, NH), 12.79 (b rs, 1H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 33.7 (CH), 36.0 (CH2), 50.5 (NCH2), 107.0, 116.5, 122.3, 127.4, 132.6, 134.4 (Ar-C), 153.6, 172.0, 173.9 (C=O); HRMS (ESI): m/z calcd for C12H10ClN2O5 297.0278 [M + H]+, found 297.0273.
Methyl 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylate (8): To 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylic acid (1) (12.75 g, 0.05 mol) completely dissolved in methanol (100 mL), H2SO4 (3 mL) was added, a reaction mixture was heated at reflux for 18 h. After cooling the reaction mixture down, aqueous 5% Na2CO3 solution was added until pH 7–8. Precipitate was filtered off, washed with water, and recrystallized from methanol. Yield 68%; white solid; m.p. 145−146 °C; IR (KBr) νmax (cm−1): 3059 (OH), 1738, 1659 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 2.64–2.72 (m, 2H, COCH2), 3.48–3.52 (m, 1H, CH), 3.70 (s, 3H, CH3), 3.86–3.94 (m, 2H, NCH2), 6.93 (d, J = 9.1 Hz, 1H, Ar-H), 7.19 (d, J = 9.1 Hz, 1H, Ar-H), 7.24 (s, 1H, Ar-H), 9.97 (s, 1H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 33.4 (CH), 36.0 (CH2), 50.4 (NCH2), 52.1 (OCH3), 118.1, 121.9, 126.5, 127.9, 151.8 (Ar-C), 172.0, 173.1 (C=O); HRMS (ESI): m/z calcd for C12H13ClNO4 270.0533 [M + H]+, found 270.0542.
1-(5-Chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (9): To methyl 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carboxylate (8) (8.07 g, 0.03 mol) completely dissolved in propan-2-ol (80 mL), hydrazine hydrate (1.92 g, 1.87 mL, 0.06 mol) was added. The reaction mixture was heated at reflux for 1.5 h. Precipitate was filtered off, washed with water, and recrystallized from methanol. Yield 66%; white solid; m.p. 193–194 °C; IR (KBr) νmax (cm−1): 3360, 3302 (NH2), 3078 (OH), 2929 (NH), 1695, 1636 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 2.53–2.60 (m, 2H, COCH2), 3.18–3.23 (m, 1H, CH), 3.69–3.83 (m, 2H, NCH2), 4.32 (s, 2H, NH2), 6.92 (d, J = 9.1 Hz, 1H, Ar-H), 7.18 (d, J = 9.1 Hz, 1H, Ar-H), 7.22 (s, 1H, Ar-H), 9.28 (s, 1H, NH), 9.95 (s, 1H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 34.2 (CH), 35.6 (CH2), 51.3 (NCH2), 118.0, 121.9, 126.6, 128.0, 151.8 (Ar-C), 171.6, 172.5 (C=O); HRMS (ESI): m/z calcd for C11H13ClN3O3 270.0645 [M + H]+, found 270.0645.
1-(5-Chloro-2-hydroxyphenyl)-4-(5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)pyrrolidin-2-one (10): To KOH (0.45 g, 8 mmol) dissolved in ethanol (25 mL), CS2 (0.91 g, 0.73 mL, 12 mmol) was added and a reaction mixture was stirred at room temperature for 15 min. Afterwards, 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (9) (1.08 g, 4 mmol) dissolved in ethanol (25 mL) was added and a reaction mixture was heated at reflux for 4 h. After solvent evaporation, the residue was dissolved in water (20 mL) and hydrochloric acid was added to pH 3–4. Liquid fractions were removed under vacuo and the residue was diluted with water. Precipitate was filtered off, washed with water, and recrystallized from methanol. Yield 79 %; white solid; m.p. 127–128 °C; IR (KBr) νmax (cm−1): 3430 (OH), 2924 (NH), 1635 (C=O), 1118 (C=S); 1H-NMR (DMSO-d6, 400 MHz) δ: 2.59–2.66 (m, 2H, COCH2), 3.81–3.91 (m, 3H, CH+NCH2), 6.95–7.21 (m, 3H, Ar-H), 10.10 (s, 1H, OH), 13.56 (s, 1H, NH); 13C-NMR (DMSO-d6, 101 MHz) δ: 33.5 (CH), 36.2 (CH2), 50.6 (NCH2), 118.1, 121.8, 126.5, 127.9, 151.9 (Ar-C), 164.1 (C=N), 172.4 (C=O), 174.2 (C=S); HRMS (ESI): m/z calcd for C12H11ClN3O3S 312.0209 [M + H]+, found 312.0731.
1-(5-Chloro-2-hydroxyphenyl)-N-(1,3-dioxoisoindolin-2-yl)-5-oxopyrrolidine-3-carboxamide (11): To 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (9) (1.08 g, 4 mmol) dissolved in 1,4-dioxane (5 mL), phthalic anhydride (0.59 g, 4 mmol) was added and a reaction mixture was heated at reflux for 4 h. Precipitate was filtered off, washed with water, and recrystallized from propan-2-ol. Yield 87%; pink solid; m.p. 263–264 °C; IR (KBr) νmax (cm−1): 3267 (OH), 3016 (NH), 1657, 1678, 1750 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 2.63–2.68 (m, 1H, COCH2), 2.75–2.79 (m, 1H, COCH2), 3.59 (qui, J = 4.6 Hz, 1H, CH), 3.80–3.84 (m, 1H, NCH2), 4.01–4.04 (m, 1H, NCH2), 6.93–7.24 (m, 3H, Ar-H), 7.93–8.00 (4H, m, Ar-H), 10.00 (s, 1H, NH), 10.96 (s, 1H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 33.7 (CH); 35.0 (CH2); 50.9 (NCH2), 118.0, 121.9; 123.8, 126.4, 128.0, 129.5, 135.3, 151.8 (Ar-C), 165.0, 171.9, 171.9 (C=O); HRMS (ESI): m/z calcd for C19H15ClN3O5 400.0700 [M + H]+, found 400.0693.
1-(5-Chloro-2-hydroxyphenyl)-N-(2,5-dimethyl-1H-pyrrol-1-yl)-5-oxopyrrolidine-3-carboxamide (12): A mixture of 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (9) (1.08 g, 4 mmol), hexane-2,5-dione (0.46 g, 0.47 mL, 4 mmol) propan-2-ol (40 mL), and glacial acetic acid (1 mL) was heated at reflux for 2 h. The reaction mixture was diluted with water (50 mL) and heated again up to boiling point. Precipitate formed after cooling down was filtered off, washed with water, and recrystallized from propan-2-ol. Yield 84%; white solid; m.p. 203–204 °C; IR (KBr) νmax (cm−1): 3261 (NH), 3089 (OH), 1666, 1594 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 1.98, 2.00 (2s, 6H, 2CH3), 2.68–2.74 (m, 2H, COCH2), 3.50 (qui, J = 4.0 Hz, 1H, CH), 3.85–4.02 (m, 2H, NCH2), 5.65 (s, 2H, 2CH), 6.92–7.26 (m, 3H, Ar-H), 9.99 (s, 1H, NH), 10.86 (s, 1H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 10.9 (CH3), 33.6 (CH), 35.3 (CH2), 51.1 (NCH2), 103.1 (CH), 118.0, 121.9, 126.4, 127.9, 151.8 (Ar-C), 171.7, 172.2 (C=O); HRMS (ESI): m/z calcd for C17H19ClN3O3 348.1115 [M + H]+, found 348.1117.
1-(5-Chloro-2-hydroxyphenyl)-4-(3,5-dimethyl-1H-pyrazole-1-carbonyl)pyrrolidin-2-one (13): Method A. To 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (9) (1.08 g, 4 mmol) dissolved in hot propan-2-ol (25 mL), pentane-2,4-dione (0.82 mL, 8 mmol) and HCl (2-3 drops) were added. A mixture was heated at reflux for 5 h. Liquid fractions were removed under vacuo, the residue was poured over with water (100 mL), heated to the boiling point until complete dissolution. Precipitate formed after cooling down was filtered off, washed with water, and recrystallized from propan-2-ol. Method B. A mixture of 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (9) (0.20 g, 0.75 mmol), propan-2-ol (5 mL), and pentane-2,4-dione (0.15 mL, 1.5 mmol) was subjected to microwave irradiation at 100 W at 100 °C for 20 min. Liquid fractions were removed under vacuo, the residue was diluted with water (30 mL), and heated to the boiling point. Precipitate formed after cooling down was filtered off, washed with water, and recrystallized from propan-2-ol. Yield A 82%; B 60%; white solid; m.p. 99–100 °C; IR (KBr) νmax (cm−1): 3130 (OH), 1724, 1662 (C=O); 1H-NMR (DMSO-d6, 400 MHz) δ: 1.20, 1.21 (2s, 6H, 2CH3), 2.55–2.71 (m, 2H, COCH2), 3.78–3.94 (m, 3H, CH+NCH2), 4.90–5.00 (m, 1H, CH), 6.90–7.21 (m, 3H, Ar-H), 10.00 (s, 1H, OH); 13C-NMR (DMSO-d6, 101 MHz) δ: 33.4 (CH), 36.0 (CH2), 50.4 (NCH2), 52.1 (CH3), 108.7, 118.0, 121.9, 126.4, 127.9, 144.1, 151.4, 151.8 (Ar-C), 172.0, 173.1 (C=O); HRMS (ESI): m/z calcd for C16H17ClN3O3 334.0958 [M + H]+, found 334.0521.
3.2.1. General Procedure for Synthesis of 14–17
To 1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbohydrazide (9) (1.08 g, 4 mmol) dissolved in methanol (25 mL), corresponding cyanate (6 mmol) was added and a reaction mixture was heated at reflux for 10 min – 4 h. Precipitate was filtered off and recrystallized from methanol.
2-(1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbonyl)-N-phenylhydrazine-1-carboxamide (14): Prepared from phenyl isocyanate (0.66 mL, 6 mol) by heating a reaction mixture at reflux for 2 h. Yield 91%; white solid; m.p. 139–140 °C; IR (KBr) νmax (cm−1): 3273 (OH), 3040, 2984, 2966 (NH), 1691, 1671, 1642 (C=O); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.64–2.67 (m, 2H, COCH2), 3.34–3.40 (m, 1H, CH), 3.72–3.96 (m, 2H, NCH2), 6.89–7.47 (m, 8H, Ar-H), 8.10 (s, 1H, NH), 8.78 (s, 1H, NH), 9.91 (s, 1H, OH), 9.95 (s, 1H, NH); 13C-NMR (DMSO-d6, 75 MHz) δ: 33.9 (CH), 35.3 (CH2), 51.1 (NCH2), 118.0, 118.5, 121.9, 126.6, 127.9, 127.9, 128.7, 139.5, 151.8 (Ar-C), 155.2, 172.3, 172.4 (C=O); HRMS (ESI): m/z calcd for C18H18ClN4O4 389.1016 [M + H]+, found 389.1013.
2-(1-(5-Chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbonyl)-N-(4-chlorophenyl)hydrazine-1-carboxamide (15): Prepared from 4-chlorophenyl isocyanate (0.77 g, 6 mmol) by heating a reaction mixture at reflux for 10 min. Yield 93%; white solid; m.p. 199–200 °C; IR (KBr) νmax (cm−1): 3273 (OH), 3040, 2984, 2966 (NH), 1691, 1671, 1642 (C=O); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.60–2.67 (m, 2H, COCH2), 3.34–3.40 (m, 1H, CH), 3.73–3.96 (m, 2H, NCH2), 6.91–7.52 (m, 7H, Ar-H), 8.19 (s, 1H, NH), 8.94 (s, 1H, NH), 9.92 (s, 1H, OH), 9.95 (s, 1H, NH); 13C-NMR (DMSO-d6, 75 MHz) δ: 33.9 (CH), 35.3 (CH2), 51.7 (NCH2), 118.0, 119.6, 120.0, 121.9, 126.6, 127.9, 127.9, 128.6, 138.2, 138.6, 151.7 (Ar-C), 153.9, 172.3, 172.4 (C=O); HRMS (ESI): m/z calcd for C18H17Cl2N4O4 423.0627 [M + H]+, found 423.0619.
2-(1-(5-Chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbonyl)-N-phenylhydrazine-1-carbothioamide (16): Prepared from phenyl isothiocyanate (0.72 mL, 6 mmol) by heating a reaction mixture at reflux for 3 h. Yield 88%; white solid; m.p. 138–139 °C; IR (KBr) νmax (cm−1): 3174 (OH), 3018, 2971, 2883 (NH), 1677, 1662 (C=O), 1206 (C=S); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.62–2.70 (m, 2H, COCH2), 3.79–4.01 (m, 3H, CH+NCH2), 6.93–7.46 (m, 8H, Ar-H), 9.60 (s, 1H, NH), 10.01(s, 1H, OH), 10.19, 11.14 (2s, 2H, 2NH); 13C-NMR (DMSO-d6, 75 MHz) δ: 33.9 (CH), 36.2 (CH2), 51.1 (NCH2), 118.1, 121.9, 126.6, 127.9, 128.0, 128.9, 130.0, 139.1, 151.8 (Ar-C), 172.2, 172.4 (C=O), 182.5 (C=S); HRMS (ESI): m/z calcd for C18H18ClN4O3S 405.0788 [M + H]+, found 405.0784.
2-(1-(5-Chloro-2-hydroxyphenyl)-5-oxopyrrolidine-3-carbonyl)-N-methylhydrazine-1-carbothioamide (17): Prepared from methyl isothiocyanate (0.44 g, 0.41 mL, 6 mmol) by heating a reaction mixture at reflux for 4 h. Yield 71%; white solid; m.p. 249–250 °C; IR (KBr) νmax (cm−1): 3172 (OH), 3056, 2972, 2782 (NH), 1652, 1494 (C=O), 1255 (C=S); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.70–2.91 (m, 2H, COCH2), 3.45 (s, 3H, CH3), 3.90–4.11 (m, 3H, CH+NCH2), 6.92–7.26 (m, 3H, Ar-H), 8.18 (s, 1H, NH), 9.66 (s, 1H, NH), 10.01 (s, 1H, OH), 10.24 (s, 1H, NH); 13C-NMR (DMSO-d6, 75 MHz) δ: 28.8 (CH3), 30.0 (CH), 34.1 (CH2); 51.1 (NCH2), 118.1, 121.9, 126.5, 128.0, 151.8 (Ar-C), 152.9 (C=O), 167.4 (C=S), 172.0 (C=O); HRMS (ESI): m/z calcd for C13H16ClN4O3S 343.0631 [M + H]+, found 343.0740.
1-(5-Chloro-2-hydroxyphenyl)-4-(5-(methylamino)-1,3,4-thiadiazol-2-yl)pyrrolidin-2-one (18): To conc. H2SO4 (5 mL) carbothioamide 17 (0.68 g, 2 mmol) was added in portions and a reaction mixture was stirred at room temperature for 3 h. Afterwards, it was added dropwise into water-ice mixture. Precipitate was filtered off and recrystallized from methanol. Yield 62%; white solid; m.p. 249–250 °C; IR (KBr) νmax (cm−1): 3172 (OH), 3055, 2970 (NH), 1652 (C=O); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.72–2.90 (m, 2H, COCH2), 3.45 (s, 3H, CH3), 3.85–4.15 (m, 3H, CH+NCH2), 6.88–7.30 (m, 3H, Ar-H), 9.97 (s, 1H, OH), 13.64 (s, 1H, NH); 13C-NMR (DMSO-d6, 75 MHz) δ: 28.8 (CH3), 29.9 (CH), 34.1 (CH2), 51.1 (NCH2), 118.1, 121.9, 126.4, 127.9, 128.0, 151.8, 152.9, 167.4 (Ar-C), 171.9 (C=O); HRMS (ESI): m/z calcd for C13H14ClN4O2S 325.0526 [M + H]+, found 325.0519.
3.2.2. General Procedure for Synthesis of 19–21
A mixture of a corresponding carboxamide (2 mmol) and aqueous 10% KOH solution (25 mL) was heated at reflux for 12 h. After cooling down, 10% HCl solution was added to pH 5. Precipitate was filtered off and recrystallized from DMF/H2O mixture.
5-(1-(5-Chloro-2-hydroxyphenyl)-5-oxopyrrolidin-3-yl)-4-phenyl-2,4-dihydro-3H-1,2,4-triazol-3-one (19): Prepared from carboxamide 14 (0.78 g, 2 mmol). Yield 71%; white solid; m.p. 124–125 °C; IR (KBr) νmax (cm−1): 3195 (OH), 1704, 1695 (C=O); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.65–2.93 (m, 2H, COCH2), 3.85–4.14 (m, 3H, CH+NCH2), 6.86–7.30 (m, 8H, Ar-H), 9.99 (s, 1H, OH), 13.63 (s, 1H, NH); 13C-NMR (DMSO-d6, 75 MHz) δ: 33.9 (CH), 34.4 (CH2), 50.9 (NCH2), 118.0, 121.8, 126.3, 127.8, 128.8, 132.6, 137.8, 142.8, 151.7, 154.4 (Ar-C), 171.8, 172.9 (C=O); HRMS (ESI): m/z calcd for C18H16ClN4O3S 371.0911 [M + H]+, found 371.0907.
1-(5-Chloro-2-hydroxyphenyl)-4-(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)pyrrolidin-2-one (20): Prepared from carbothioamide 16 (0.81 g, 2 mmol). Yield 90%; white solid; m.p. 266–267 °C; IR (KBr) νmax (cm−1): 3069 (OH), 3017 (NH), 1665 (C=O), 1276 (C=S); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.69–2.78 (m, 2H, COCH2), 3.57–3.65 (m, 2H, NCH2), 3.80–3.88 (m, 1H, CH), 6.87–7.64 (m, 8H, Ar-H), 9.94 (s, 1H, OH), 13.88 (s, 1H, NH); 13C-NMR (DMSO-d6, 75 MHz) δ: 29.1 (CH), 34.4 (CH2), 51.1 (NCH2), 118.0, 121.9, 126.2, 128.0, 128.6, 129.7, 129.8, 133.6, 151.8, 152.7 (Ar-C), 168.4 (C=O), 171.7 (C=S); HRMS (ESI): m/z calcd for C18H16ClN4O2S 387.0678 [M + H]+, found 387.0679.
1-(5-Chloro-2-hydroxyphenyl)-4-(4-methyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)pyrrolidin-2-one (21): Prepared from carbothioamide 17 (0.68 g, 2 mmol). Yield 72%; white solid; m.p. 244–245 °C; IR (KBr) νmax (cm−1): 3169 (OH), 2971 (NH), 1652 (C=O), 1254 (C=S); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.65–2.91 (m, 2H, COCH2), 3.46 (s, 3H, CH3), 3.80–4.14 (m, 3H, CH+NCH2), 6.91–7.24 (m, 3H, Ar-H), 9.98 (s, 1H, OH), 13.64 (s, 1H, NH); 13C-NMR (DMSO-d6, 75 MHz) δ: 28.8 (CH3), 29.9 (CH), 34.1 (CH2), 51.1 (NCH2), 118.1, 121.9, 126.4, 127.9, 151.8, 152.9 (Ar-C), 167.4 (C=O), 171.9 (C=S); HRMS (ESI): m/z calcd for C13H14ClN4O2S 325.0526 [M + H]+, found 325.0523.
3.2.3. General procedure for synthesis of 22–26
To 1-(5-chloro-2-hydroxyphenyl)-4-(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)pyrrolidin-2-one (20) (0.77 g, 2 mmol) dissolved in DMF (5 mL), triethylamine (0.51 g, 0.70 mL, 5 mmol) and haloalkane (3 mmol) were added. The reaction mixture was stirred at room temperature for 4 h and then diluted with cold water (30 mL). Precipitate was filtered off and recrystallized from propan-2-ol (unless indicated otherwise).
1-(5-Chloro-2-hydroxyphenyl)-4-(5-(ethylthio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyrrolidin-2-one (22): Prepared from iodoethane (0.47g, 0.24 mL, 3 mmol). Yield 69%; white solid; m.p. 161–162 °C; IR (KBr) νmax (cm−1): 3061 (OH), 1695 (C=O); 1H-NMR (DMSO-d6, 300 MHz) δ: 1.29 (t, J = 7.2 Hz, 3H, CH3), 2.52–2.59 (m, 1H, COCH2); 2.72–2.81 (m, 1H, CH2); 3.08 (q, J = 7.2 Hz, 2H, CH3CH2), 3.63–3.98 (m, 2H, CH+NCH2), 6.89–7.65 (m, 8H, Ar-H), 10.12 (s, 1H, OH); 13C-NMR (DMSO-d6, 75 MHz) δ: 14.8 (CH3), 26.5 (CH2CH3), 28.8 (CH), 35.3 (CH2), 52.0 (NCH2), 118.1, 121.8, 126.3, 127.6, 128.1, 130.0, 130.2, 132.8, 150.9, 152.0, 156.4 (Ar-C), 171.9 (C=O); HRMS (ESI): m/z calcd for C20H20ClN4O2S 415.0995 [M + H]+, found 415.0999.
Ethyl 2-((5-(1-(5-chloro-2-hydroxyphenyl)-5-oxopyrrolidin-3-yl)-4-phenyl-4H-1,2,4-triazol-3-yl)thio)acetate (23): Prepared from ethyl chloroacetate (0.37 g, 0.32 mL, 3 mmol). Yield 82%; white solid; m.p. 174–175 °C; IR (KBr) νmax (cm−1): 3068 (OH), 1749, 1670 (C=O); 1H-NMR (DMSO-d6, 300 MHz) δ: 1.18 (t, J = 7.2 Hz, 3H, CH3CH2), 2.53–2.78 (m, 2H, COCH2), 3.35 (s, 1H, CH), 3.65–3.80 (m, 2H, NCH2), 3.89–3.97 (m, 1H, CH), 4.11 (q, J = 7.2 Hz, J = 14.1 Hz, 2H, CH3CH2), 4.06 (s, 2H, CH2), 4.09–4.17 (m, 2H, CH2), 6.87–7.68 (m, 8H, Ar-H), 10.07 (s, 1H, OH); 13C-NMR (DMSO-d6, 75 MHz) δ: 14.0 (CH3), 28.8 (CH2CH3), 33.9 (CH), 35.3 (CH2), 52.0 (NCH2), 61.3 (CH3CH2), 118.1, 121.8, 126.3, 127.5, 128.0, 130.1, 130.4, 132.5, 150.2, 151.9, 156.5 (Ar-C), 168.0, 171.8 (C=O); HRMS (ESI): m/z calcd for C22H22ClN4O4S 473.1050 [M + H]+, found 473.1054.
1-(5-Chloro-2-hydroxyphenyl)-4-(5-((2-oxo-2-phenylethyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyrrolidin-2-one (24): Prepared from 2-bromo-1-phenylethanone (0.60 g, 3 mmol). Yield 87%; white solid; m.p. 99–100 °C (propan-2-ol/H2O); IR (KBr) νmax (cm−1): 3063 (OH), 1695, 1680 (C=O); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.53–2.78 (m, 2H, COCH2), 3.35 (s, 1H, CH), 3.58–3.96 (m, 2H, NCH2), 4.89 (s, 2H, CH2), 6.87–8.02 (m, 13H, Ar-H), 10.08 (s, 1H, OH); 13C-NMR (DMSO-d6, 75 MHz) δ: 28.8 (CH), 35.3 (CH2), 40.1 (CH2CO), 52.0 (NCH2), 118.1, 121.8, 126.2, 127.5, 128.0, 128.4, 128.8, 130.1, 130.4, 132.6, 133.7, 135.3, 150.5, 151.9, 156.4 (Ar-C), 171.8, 193.0 (C=O); HRMS (ESI): m/z calcd for C26H22ClN4O3S 505.1101 [M + H]+, found 505.1095.
1-(5-Chloro-2-hydroxyphenyl)-4-(5-((2-(4-chlorophenyl)-2-oxoethyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyrrolidin-2-one (25): Prepared from 2-bromo-1-(4-chlorophenyl)ethan-1-one (0.70 g, 3 mmol). Yield 83%; white solid; m.p. 69–70 °C; IR (KBr) νmax (cm−1): 3064 (OH), 1699, 1678 (C=O); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.53–2.77 (m, 2H, COCH2), 3.65–3.79 (m, 2H, NCH2), 3.87–3.95 (m, 1H, CH), 4.87 (s, 2H, CH2), 6.84–8.07 (m, 12H, Ar-H), 10.04 (s, 1H, OH); 13C-NMR (DMSO-d6, 75 MHz) δ: 28.8 (CH), 35.3 (CH2), 40.0 (CH2CO), 52.0 (NCH2), 118.1, 121.8, 126.2, 127.5, 128.0, 128.9, 130.1, 130.3, 130.6, 132.5, 134.0, 138.7, 150.4, 151.9, 156.5 (Ar-C), 171.8, 192.2 (C=O); HRMS (ESI): m/z calcd for C26H21Cl2N4O3S 539.0711 [M + H]+, found 539.0706.
1-(5-Chloro-2-hydroxyphenyl)-4-(5-((2-(4-nitrophenyl)-2-oxoethyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyrrolidin-2-one (26): Prepared from 2-bromo-1-(4-nitrophenyl)ethanone (0.73 g, 3 mmol). Yield 81%; white solid; m.p. 104–105 °C; IR (KBr) νmax (cm−1): 3071 (OH), 1703, 1601 (C=O); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.41–2.48 (m, 1H, COCH2), 2.69–2.78 (m, 2H, CH+COCH2), 3.57–3.66 (m, 2H, NCH2), 3.80–3.88 (m, 2H, CH2), 6.84–7.63 (m, 12H, Ar-H), 9.91 (s, 1H, OH); 13C-NMR (DMSO-d6, 75 MHz) δ: 29.1 (CH), 34.4 (CH2), 39.9 (CH2CO), 51.0 (NCH2), 118.0, 121.8, 126.2, 127.9, 128.5, 129.6, 129.7, 133.55, 151.7, 152.6 (Ar-C), 168.4, 171.6 (C=O); HRMS (ESI): m/z calcd for C26H21ClN5O5S 550.0952 [M + H]+, found 550.0945.