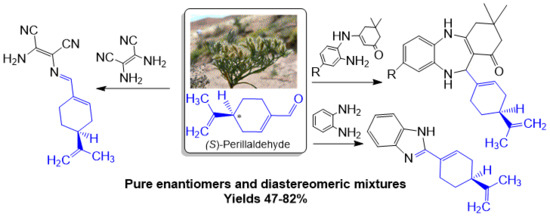
Hemi-Synthesis of Chiral Imine, Benzimidazole and Benzodiazepines from Essential Oil of Ammodaucus leucotrichus subsp. leucotrichus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry and Mechanism
2.2. Chiral HPLC and Stereochemistry
2.3. Nuclear Magnetic Resonance Spectroscopy
2.4. Single-Crystal X-ray Diffraction
3. Materials and Methods
3.1. General Remarks
3.2. Plant Material and Extraction Procedure
3.3. General Procedure for the Hemi-Synthesis of Compounds 1–4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Appendino, G.; Minassi, A.; Taglialatela-Scafati, O. Recreational drug discovery: natural products as lead structures for the synthesis of smart drugs. Nat. Prod. Rep. 2014, 31, 880–904. [Google Scholar] [CrossRef] [PubMed]
- Bonikowski, R.; Kula, J.; Bujacz, A.; Wajs-Bonikowska, A.; Zaklos-Szyda, M.; Wysocki, S. Hydroindene-derived chiral synthons from carotol and their cytotoxicity. Tetrahedron Asymm. 2012, 23, 1038–1045. [Google Scholar] [CrossRef]
- Huangyong, L.; Changshui, C.; Cao, X. Essential oils-oriented chiral esters as potential pesticides: Asymmetric syntheses, characterization and bio-evaluation. Ind. Crops Prod. 2015, 76, 432–436. [Google Scholar]
- Sakirigui, A.; Gbaguidi, F.; Kasséhin, U.; JacquesPoupaert; Accrombessi, G.; Kotchoni, S. Structural and antitrypanosomal data of different carbasones of piperitone. Data Brief 2016, 9, 1039–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Pérez de Paz, P.L.; Palá-Paúl, J.; Sanz, J. Analysis by gas chromatography–mass spectrometry of the volatiles from the fruits of Ammodaucus leucotrichus subsp. leucotrichus and subsp. nanocarpus grown in North Africa and the Canary Islands, respectively. J. Chromatogr. A 2006, 1108, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Anthelme, F.; Waziri Mato, M.; Maley, J. Elevation and local refuges ensure persistence of mountain specific vegetation in the Nigerien Sahara. J. Arid Environ. 2008, 72, 2232–2242. [Google Scholar] [CrossRef]
- Jouad, H.; Haloui, M.; Rhiouani, H.; El Hilaly, J.; Eddouks, M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez–Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef]
- Merzouki, A.; Ed-derfoufi, F.; Molero Mesa, J. Contribution to the knowledge of Rifian traditional medicine. II: Folk medicine in Ksar Lakbir district (NW Morocco). Fitoterapia 2000, 71, 278–307. [Google Scholar] [CrossRef]
- Hammiche, V.; Maiza, K. Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Dahmane, D.; Dob, T.; Krimat, S.; Nouasri, A.; Metidji, H.; Ksouri, A. Chemical composition, antioxidant and antibacterial activities of the essential oils of medicinal plant Ammodaucus leucotrichus from Algeria. J. Essent. Oil Res. 2017, 29, 48–55. [Google Scholar] [CrossRef]
- Khaldi, A.; Meddah, B.; Moussaoui, A.; Sonnet, P. Anti-mycotoxin Effect and Antifungal Properties of Essential Oil from Ammodaucus leucotrichus Coss. & Dur. on Aspergillus flavus and Aspergillus ochraceus. J. Essent. Oil Bear. 2017, 20, 36–44. [Google Scholar]
- Abu Zarga, M.H.; Al-Jaber, H.I.; Baba Amer, Z.Y.; Sakhrib, L.; Al-Qudah, M.A.; Al-humaidi, J.Y.G.; Abaza, I.F.; Afifi, F.U. Chemical Composition, Antimicrobial and Antitumor Activities of Essential Oil of Ammodaucus leucotrichus Growing in Algeria. JAPN 2013, 3, 224–231. [Google Scholar]
- Mayeku, W.P.; Omollo, N.I.; Odalo, O.J.; Hassanali, A. Chemical composition and mosquito repellency of essential oil of Conyza newii propagated in different geographical locations of Kenya. Med. Vet. Entomol. 2014, 28, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wang, S.Y.; Chen, C. Increasing Antioxidant Activity and Reducing Decay of Blueberries by Essential Oils. J. Agric. Food Chem. 2008, 56, 3587–3592. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zeng, X.; Lü, A.; Zhu, A.; Peng, X.; Wang, Y. Perillaldehyde, a potential preservative agent in foods: Assessment of antifungal activity against microbial spoilage of cherry tomatoes. Food Sci. Technol. 2015, 60, 63–70. [Google Scholar] [CrossRef]
- Appelt, H.R.; Oliveira, J.S.; Santos, R.C.V.; Rodrigues, O.E.D.; Santos, M.Z.; Heck, E.F.; Rosa, L.C.R. Synthesis and Antimicrobial Activity of Carbohydrate Based Schiff Bases: Importance of Sugar Moiety. Int. J. Carbohydr. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Kundu, A.; Shakil, N.A.; Saxena, D.B.; Pankaj; Kumar, J.; Walia, S. Microwave assisted solvent-free synthesis and biological activities of novel imines (Schiff bases). J. Environ. Sci. Health 2009, 44, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Sonnekar, V.S.; Jadhav, W.N.; Dake, D.S.; Pawar, R. Synthesis and antimicrobial and antifungal activities of novel bis-imine derivatives. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1411–1418. [Google Scholar]
- Missaoui, B.E.; Ouahrani, M.R.; Kouadri, Y.; Chebrouk, F.; Gherraf, N. Synthesis of Novel Heterocyclic Compounds Containing 1,5-Benzodiazepine. Asian J. Chem. 2015, 27, 2175–2177. [Google Scholar] [CrossRef]
- Kolos, N.N.; Yurchenko, E.N.; Orlov, V.D.; Shishkina, S.V.; Shishkin, O.V. Investigation of the products of interaction of cyclic diketones with nitrogen-containing 1,4-binucleophiles. Chem. Heterocycl. Compd. 2004, 40, 1550–1559. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, P.; Sachar, A.; Sharma, R.L. Facile and one pot synthetic routes for various novel, differently fused and promising heteropolycycles. J. Heterocycl. Chem. 2010, 47, 334–349. [Google Scholar]
- Cherfaoui, B.; Lakhdari, H.; Bennamane, N.; Ameraoui, R.; Talhi, O.; Almeida Paz, F.A.; Bachari, K.; Kirsch, G.; Nejar-Bellara, K.; Silva, A.M.S. Dibenzo[b,e][1,4]diazepin-1-ones and their Ring-Opened Derivatives: Revisited Synthesis, 2D NMR and Crystal Structure. Synlett 2017, 28, 2247–2252. [Google Scholar]
- Ling, I.; Podanyi, B.; Hamori, T.; Solyom, S. Asymmetric reduction of a carbon-nitrogen double bond: enantioselective synthesis of 4,5-dihydro-3H-2,3-benzodiazepines. J. Chem. Soc. Perkin Trans. 1 1995, 11, 1423–1427. [Google Scholar] [CrossRef]
- Al-Azmi, A.; Elassara, A.A.; Booth, B.L. The chemistry of diaminomaleonitrile and its utility in heterocyclic synthesis. Tetrahedron 2003, 59, 2749–2763. [Google Scholar] [CrossRef]
- Grell, J.; Bernstein, J.; Tinhofer, G. Graph-set analysis of hydrogen-bond patterns: some mathematical conceptsWork supported by grant No. I-0333-263.06/93 from the GIF, the German-Israeli Foundation for Scientific Research and Development. Acta Crystallogr. Sect. B Struct. Sci. 1999, 55, 1030–1043. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebrouk, F.; Madani, K.; Cherfaoui, B.; Boukenna, L.; Válega, M.; Mendes, R.F.; Paz, F.A.A.; Bachari, K.; Talhi, O.; Silva, A.M.S. Hemi-Synthesis of Chiral Imine, Benzimidazole and Benzodiazepines from Essential Oil of Ammodaucus leucotrichus subsp. leucotrichus. Molecules 2019, 24, 975. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050975
Chebrouk F, Madani K, Cherfaoui B, Boukenna L, Válega M, Mendes RF, Paz FAA, Bachari K, Talhi O, Silva AMS. Hemi-Synthesis of Chiral Imine, Benzimidazole and Benzodiazepines from Essential Oil of Ammodaucus leucotrichus subsp. leucotrichus. Molecules. 2019; 24(5):975. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050975
Chicago/Turabian StyleChebrouk, Farid, Khodir Madani, Brahim Cherfaoui, Leila Boukenna, Mónica Válega, Ricardo F. Mendes, Filipe A. A. Paz, Khaldoun Bachari, Oualid Talhi, and Artur M. S. Silva. 2019. "Hemi-Synthesis of Chiral Imine, Benzimidazole and Benzodiazepines from Essential Oil of Ammodaucus leucotrichus subsp. leucotrichus" Molecules 24, no. 5: 975. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050975