Screen-printed Microsensors Using Polyoctyl-thiophene (POT) Conducting Polymer As Solid Transducer for Ultratrace Determination of Azides
Abstract
:1. Introduction
2. Results
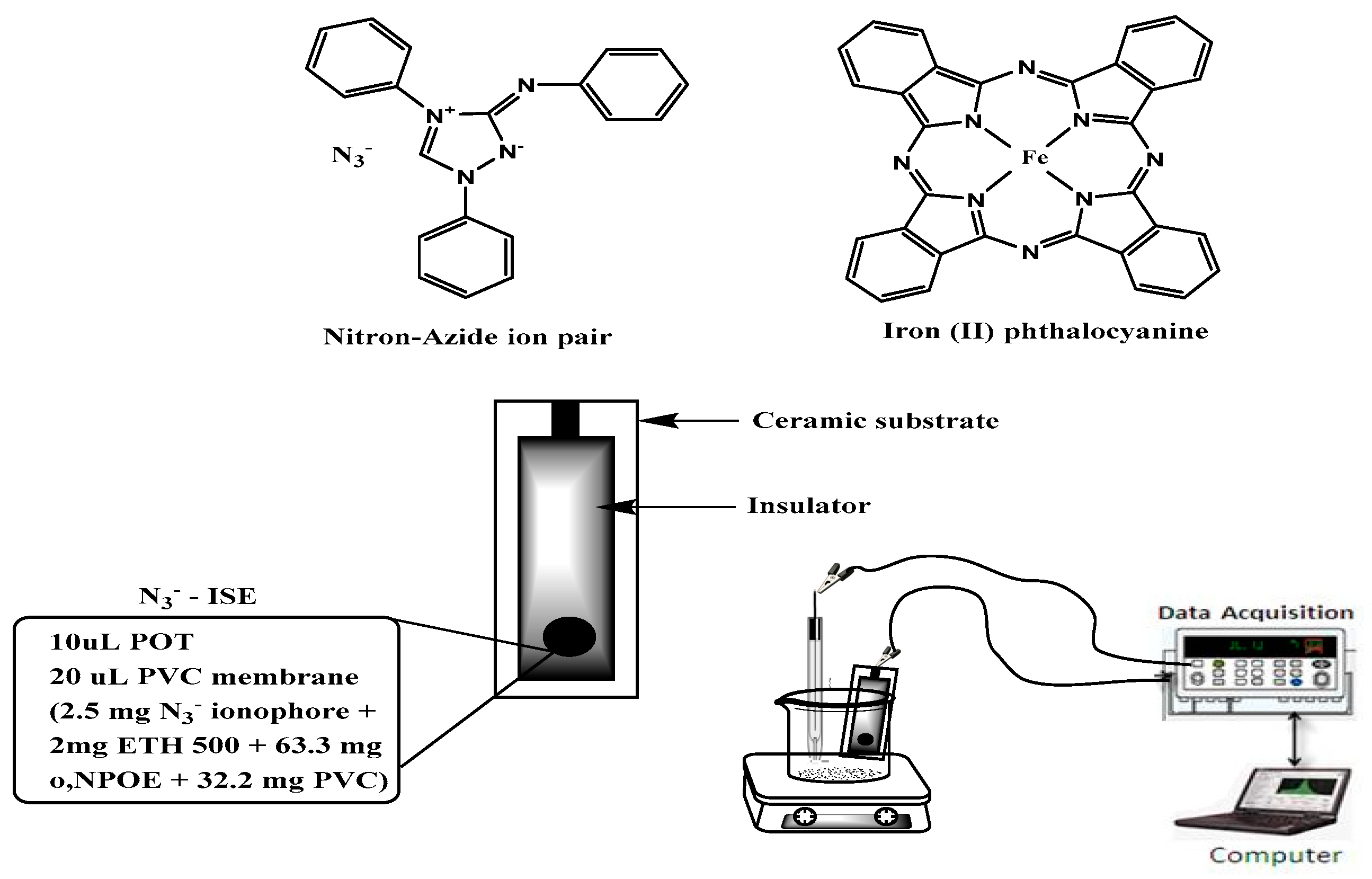
2.1. Sensors Construction and Characteristics
2.2. Robustness
2.3. Sensors’ Selectivity
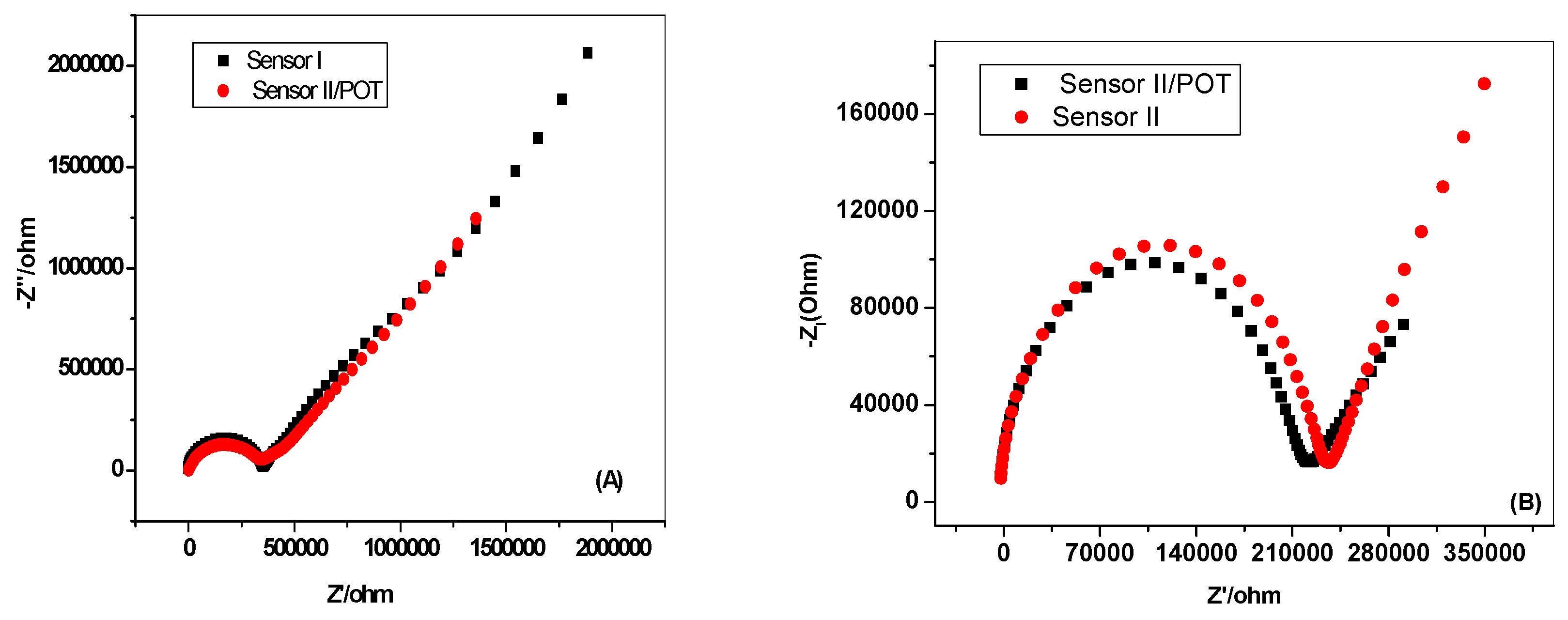
2.4. Electrochemical Impedance Spectroscopy (EIS) Measurements
2.5. Chronopotentiometric Measurements
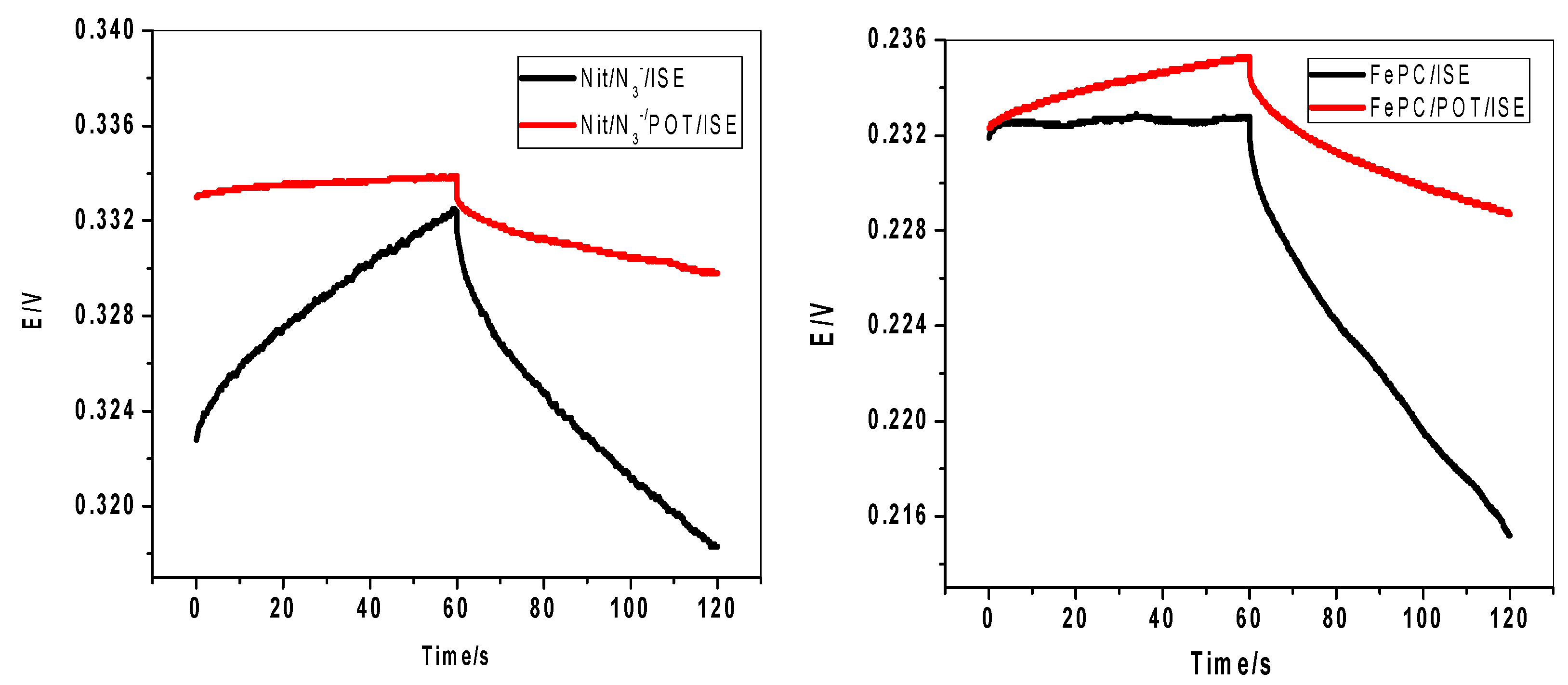
2.6. Effect of Water Film Test of the Electrode Potential
2.7. Analytical Applications
3. Materials and Methods
3.1. Apparatus
3.2. Reagents and Materials
3.3. Nitron-azide Ion-pair Complex
3.4. Sensor Fabrication
3.5. Sensor Calibration and Selectivity Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Mikhelson, K.N. Ion-Selective Electrodes; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bieg, C.; Fuchsberger, K.; Stelzle, M. Introduction to polymer-based solid-contact ion-selective electrodes—Basic concepts, practical considerations, and current research topics. Anal. Bioanal. Chem. 2017, 409, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Cadogan, A.; Gao, Z.; Lewenstam, A.; Ivaska, A.; Diamond, D. All-solid state sodium-selective electrode based on a calixareneionophore in a poly(vinyl chloride) membrane with a polypyrrole solid contact. Anal. Chem. 1992, 64, 2496–2501. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Buhlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Sokalski, T.; Ceresa, A.; Zwickl, T.; Pretsch, E. Large improvement of the lower detection limit of ion-selective polymer membrane electrodes. J. Am. Chem. Soc. 1997, 119, 11347–11348. [Google Scholar] [CrossRef]
- Bakker, E.; Meyerhoff, M.E. Ionophore-based membrane electrodes: New analytical concepts and non-classical response mechanisms. Anal. Chim. Acta 2000, 416, 121–137. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E. Potentiometry at trace levels. Trends Anal. Chem. 2001, 20, 11–19. [Google Scholar] [CrossRef]
- Bakker, E.; Bhakthavatsalam, V.; Gemene, K.L. Beyond potentiometry: Robust electrochemical ion sensor concepts in view of remote chemical sensing. Talanta 2008, 75, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Renedo, O.D.; Alonso-Lomillo, M.A.; Martinez, M.J.A. Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liang, R.; Yang, X.; Qin, W. Imprinted nanobead-based disposable screen-printed potentiometric sensor for highly sensitive detection of 2-naphthoic acid. Mater. Lett. 2018, 225, 138–141. [Google Scholar] [CrossRef]
- Carroll, S.; Baldwin, R.P. Self-calibrating microfabricated iridium oxide pH electrode array for remote monitoring. Anal. Chem. 2010, 82, 878–885. [Google Scholar] [CrossRef]
- Betterton, E.A. Environmental fate of sodium azide derived from automobile air bags. Crit. Rev. Environ. Sci. Technol. 2003, 33, 423–458. [Google Scholar] [CrossRef]
- Howard, J.D.; Skorgeboe, K.J.; Case, G.A.; Raysis, V.A.; Laqsina, E.Q. Death following accidental sodium azide investigation. J. Forensic Sci. 1990, 35, 193–196. [Google Scholar] [CrossRef]
- Kleinhofs, A.; Owais, W.M.; Nilan, R.A. Azide. Mutat. Res. 1978, 55, 165–195. [Google Scholar] [CrossRef]
- Chang, S.; Lamm, S.H. Human health effects of sodium azide exposure a literature review and analysis. Int. J. Toxicol. 2003, 22, 175–186. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, V.K.; Kumar, A. Metallo-tetraazaporphyrin based anion sensors: Regulation of sensor characteristics through central metal ions coordination. Anal. Chim. Acta 2004, 508, 61–70. [Google Scholar] [CrossRef]
- Watanabe, K.; Noguchi, O.; Okada, K.; Katsu, T. An azide-sensitive electrode for criminal investigation. Jpn. J. Forensic Toxicol. 1999, 17, 180–186. [Google Scholar]
- Saville, B. A Scheme for the colorimetric determination of microgram amounts of thiols. Analyst 1958, 83, 670–672. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; ElZawawy, F.M.; Marzouk, S.A.M.; Elnemma, E.M. Polyvinyl chloride matrix membrane electrodes for manual and flow injection determination of metal azides. Analyst 1992, 17, 1683–1689. [Google Scholar] [CrossRef]
- Van, J.T.; vanden Berg, C.M.G.; Martin, T.D. Gas electrode method for determination of azide in aqueous samples from there processing industry. Anal. Commun. 1997, 34, 385–387. [Google Scholar]
- Hassan, S.S.M.; Ahmed, M.A.; Marzouk, S.A.M.; Elnemma, E.M. Potentionetric gas sensor for the selective determination of azides. Anal. Chem. 1991, 63, 1547–1552. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, U.P.; Aggarwal, V.; Mehtab, S. Azide-selective sensor based on tripodal iron complex for direct azide determination in aqueous samples. Anal. Bioanal. Chem. 2008, 391, 2299–2380. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shokrollahi, A.; Montazerozohori, M.; Derki, S. Design and construction of azide carbon paste selective electrode based on a new schiff’s base complex of iron. IEEE Sens. J. 2010, 10, 814–819. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kelany, A.E.; Al-Mehrezi, S.S. Novel polymeric membrane sensors based on Mn(III) porphyrin and Co(II) phthalocyanineionophores for batch and flow injection determination of azide. Electroanalysis 2008, 20, 438–443. [Google Scholar] [CrossRef]
- Kamel, A.H. New potentiometric transducer based on a Mn(II)[2-formylquinoline thiosemicarbazone] complex for static and hydrodynamic assessment of azides. Talanta 2015, 144, 1085–1090. [Google Scholar] [CrossRef]
- Lindner, E.; Gyurcsanyi, R.E. Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. J. Solid State Electrochem. 2009, 13, 51–68. [Google Scholar] [CrossRef]
- Moreira, F.T.C.; Guerreiro, J.R.L.; Azevedo, V.L.; Kamel, A.H.; Sales, M.G.F. New biomimetic sensors for the determination of tetracycline in biological samples: Batch and flow mode operations. Anal. Methods 2010, 2, 2039–2045. [Google Scholar] [CrossRef]
- Perez, M.A.A.; Marın, L.P.; Quintana, J.C.; Pedram, M.Y. Influence of different plasticizers on the response of chemical sensors based on polymeric membranes for nitrate ion determination. Sens. Actuators B 2003, 89, 262–268. [Google Scholar] [CrossRef]
- Bakker, E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Marzouk, S.A.M.; Mohamed, A.H.K.; Badawy, N.M. Novel dicyanoargentate polymeric membrane sensors for selective determination of cyanide ions. Electroanalysis 2004, 16, 298–303. [Google Scholar] [CrossRef]
- Almeer, S.H.M.A.; Zogby, I.A.; Hassan, S.S.M. Novel miniaturized sensors for potentiometric batch and flow-injection analysis (FIA) of perchlorate in fireworks and propellants. Talanta 2014, 129, 191–197. [Google Scholar] [CrossRef]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Kamel, A.H.; Galal, H.R.; Awwad, N.S. Cost-effective and handmade paper-based potentiometric sensing platform for piperidine determination. Anal. Methods 2018, 10, 5406–5415. [Google Scholar] [CrossRef]
- Abdalla, N.S.; Youssef, M.A.; Algarni, H.; Awwad, N.S.; Kamel, A.H. All Solid-State Poly (Vinyl Chloride) Membrane Potentiometric Sensor Integrated with Nano-Beads Imprinted Polymers for Sensitive and RapidDetection of Bispyribac Herbicide as Organic Pollutant. Molecules 2019, 24, 712. [Google Scholar] [CrossRef]
- Amr, A.E.; Al-Omar, M.A.; Kamel, A.H.; Elsayed, E.A. Single-Piece Solid Contact Cu2+-Selective Electrodes Based on a Synthesized Macrocyclic Calix [4]arene Derivative as a Neutral Carrier Ionophore. Molecules 2019, 24, 920. [Google Scholar] [CrossRef]
Sample Availability: Not Available. |


| Parameter | FePC/POT | Nit-N3/POT | |||||
|---|---|---|---|---|---|---|---|
| o-NPOE | DOP | DBS | o-NPOE | DOP | DBS | ||
| Slope, (mV/decade) | −58.3 ± 0.9 | −41.3 ± 0.6 | −41.4 ± 0.2 | −55.1 ± 0.7 | −48.2 ± 0.6 | −43.6 ± 0.5 | |
| Coefficient, (r) (n=3) | −0.998 | −0.997 | −0.999 | −0.998 | −0.997 | 0.999 | |
| Detection limit, (M) | 1.0 × 10−7 | 4.3 × 10−7 | 7.2 × 10−7 | 7.7 × 10−8 | 2.1 × 10−7 | 2.4 × 10−7 | |
| Linear range, (M) | 3.5 × 10−7–1.0 × 10−2 | 1.0 × 10−6–1.0 × 10−2 | 2.6 × 10−6–1.0 × 10−2 | 1.0 × 10−7–1.0 × 10−2 | 1.0 × 10−6–1.0 × 10−2 | 1.0 × 10−6–1.0 × 10−2 | |
| Response time, (s) | <10 | <10 | <10 | <10 | <10 | <10 | |
| Working range, (pH) | 5.0–10 | 5.0–10 | 5–10 | 6.0–9 | 6.0-9 | 6.0–9 | |
| Standard deviation, (%) | 0.7 | 1.3 | 1.1 | 0.8 | 0.5 | 0.7 | |
| Accuracy, (%) | 99.6 | 99.3 | 98.8 | 98.4 | 99.5 | 99.3 | |
| Precision, (%), Cvw(%) | 1.1 | 1.2 | 1.7 | 0.7 | 1.0 | 1.2 | |
| Ionophore | Slope, (mV/decade) | Linear Range, (M) | pH Range | Detection Limit, (M) | Interference | Ref. |
|---|---|---|---|---|---|---|
| FeIII- and CoIII-complexes of 2,3,7,8,12,13,17,18-octakis (benzylthio)-5,10,15, 20-tetraazaporphyrin | −56.0 | 1.0 × 10−5– 3.5 × 10−1 | 2.3–6.4 | 1.0 × 10−6 | SCN−, ClO4−, ClO3−, NO3− | [17] |
| Cyanoaquacobyric acid heptakis (2-phenylethyl ester) | −49.0 | 5.0 × 10−5– 1.0 × 10−2 | 6.0 | - | NO2− | [18] |
| Substituted onium base salts | −57.6 | 1.0 × 10−4– 1.0 × 10−1 | 7.5–12.0 | 7.0 × 10−5 | SO42−, HCO3−, Cl−, H2PO4− | [19] |
| FeII- and NiII –bathophenan- throlineazide ion-pair complexes | −29.2 | 8.9 × 10−6– 1.0 × 10−1 | 4.3-10.5 | 8.0 × 10−7 | SCN−, S2−, NO2−, Cl− | [20] |
| Orion ammonium-sensitive gas probe model (95/12) with a Teflon semi-permeable membrane/Teflon membrane | −59.1 | 1.0 × 10−4– 1.0 × 10−1 | 1.0–3.5 | 3.5 × 10−5 | SO32−, NO2−, S2−, HCO3−, CH3COO− | [21] |
| Orion ammonium-sensitive electrode (model 95/12) with a polypropylene membrane | −58.8 | 1.0 × 10−4– 1.0 × 10−1 | 1.0–3.5 | 1.9 × 10−5 | SO32−, NO2−, S2− | [22] |
| FeIII-hydrotris-(3,5-dimethyl- pyrazolyl) borate acetylacetonate chloride | −59.4 | 6.3 × 10−7– 1.0 × 10−2 | 3.5–9.0 | 5.0 × 10−7 | - | [23] |
| FeIII- Schiff base | −58.9 | 1.0 × 10−6– 5.0 × 10−2 | 4.3–10.2 | 8.8 × 10−7 | ClO3−, IO3−, ClO4−, NO2−, NO3−, Cl−, I− | [24] |
| Mn(III)-porphyrin Co(II)-phthalocyanine | −56.3 -48.5 | 2.2 × 10−5– 1.0 × 10−2 5.1 × 10−5 – 1.0 × 10−2 | 3.9–6.5 4.2–6.5 | 1.3 × 10−5 1.7 × 10−5 | I−, CN− SO32− | [25] |
| MnII-[2-formylquinoline thiosemicarbazone] complex | −55.8 | 1.0×10−5–1.0 × 10−2 | 5.5–9.0 | 8.0 × 10−6 | - | [26] |
| Fe-PC/POT Nit-N3/POT | −58.3 −55.1 | 3.5 × 10−7– 1.0 × 10−2 3.5 × 10−7– 1.0 × 10−2 | 6.0–9.0 5.0–10.0 | 1.0 × 10−7 7.7 × 10−8 | - - | This work |
| Interfering ion | * Log Kpotx,y | |
|---|---|---|
| [FePC/POT | [Nit/N3/POT | |
| PO43− | −6.7 ± 0.2 | −6.2 ± 0.5 |
| Salicylate | −3.7 ± 0.4 | −0.5 ± 0.1 |
| NO2− | −5.27 ± 0.5 | −3.1 ± 0.3 |
| ClO4− | −3.3 ± 0.4 | −0.8 ± 0.1 |
| SCN− | −4.3 ± 0.7 | −1.1 ± 0.1 |
| I− | −3.5 ± 0.4 | −0.6 ± 0.2 |
| Cl− | −5.1 ± 0.3 | −4.7 ± 0.3 |
| Br− | −5.2 ± 0.1 | −4.9 ± 0.3 |
| SO42− | −5.6 ± 0.4 | −5.1 ± 0.4 |
| CH3COO− | −4.9 ± 0.1 | −4.2 ± 0.1 |
| NO3− | −4.2 ± 0.3 | −2.3 ± 0.5 |
| Sample | Taken, azide, mg/g | Azide, mg/ga | |||
|---|---|---|---|---|---|
| [Fe-PC/POT] | Recovery, % | [Nit-N3/POT] | Recovery, % | ||
| Mixture 1 | 1.0 | 0.98 ± 0.05 | 98.0 | 1.01 ± 0.2 | 101.0 |
| Mixture 2 | 5.0 | 5.2 ± 0.4 | 102.4 | 4.87 ± 0.1 | 97.4 |
| Mixture 3 | 10.0 | 9.93 ± 0.7 | 99.3 | 9.77 ± 0.3 | 97.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galal Eldin, A.; E. Amr, A.E.-G.; H. Kamel, A.; S. M. Hassan, S. Screen-printed Microsensors Using Polyoctyl-thiophene (POT) Conducting Polymer As Solid Transducer for Ultratrace Determination of Azides. Molecules 2019, 24, 1392. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071392
Galal Eldin A, E. Amr AE-G, H. Kamel A, S. M. Hassan S. Screen-printed Microsensors Using Polyoctyl-thiophene (POT) Conducting Polymer As Solid Transducer for Ultratrace Determination of Azides. Molecules. 2019; 24(7):1392. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071392
Chicago/Turabian StyleGalal Eldin, Ahmed, Abd El-Galil E. Amr, Ayman H. Kamel, and Saad S. M. Hassan. 2019. "Screen-printed Microsensors Using Polyoctyl-thiophene (POT) Conducting Polymer As Solid Transducer for Ultratrace Determination of Azides" Molecules 24, no. 7: 1392. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071392








