Size-Dependent Biological Effects of Quercetin Nanocrystals
Abstract
:1. Introduction
2. Results
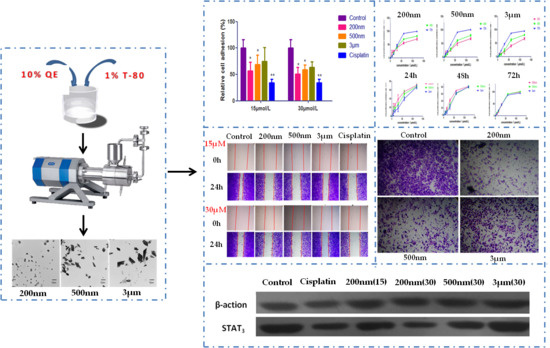
2.1. Preparation and Characterization of QE-NCs
2.2. Effect of QE-NCs on Cell Proliferation
2.3. Effect of QE-NCs on Cell Adhesion
2.4. Effect of QE-NCs on Cell Migration
2.5. Effect of QE-NCs on Cell Invasion
2.6. Effect of QE-NCs on STAT3 Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Preparation and Characterization of QE-NCs
4.2.1. Preparation of QE-NCs with Three Mean Particle Sizes
4.2.2. Characterization of QE-NCs
4.3. Cell Culture
4.4. CCK-8 Proliferation Assay
4.5. Cell Adhesion Assay
4.6. Wound Healing Assay
4.7. Matrigel Invasion Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nam, J.S.; Sharma, A.R.; Nguyen, L.T.; Chakraborty, C.; Sharma, G.; Lee, S.S. Application of bioactive quercetin in oncotherapy: From nutrition to nanomedicine. Molecules 2016, 21, 108. [Google Scholar] [CrossRef]
- Bhat, F.A.; Sharmila, G.; Balakrishnan, S.; Arunkumar, R.; Elumalai, P.; Suganya, S.; Raja Singh, P.; Srinivasan, N.; Arunakaran, J. Quercetin reverses egf-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (pc-3) cell line via egfr/pi3k/akt pathway. J. Nutr. Biochem. 2014, 25, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, E.M.; Sharma, A.R.; Ganbold, B.; Park, J.; Sharma, G.; Kang, Y.H.; Song, D.K.; Lee, S.S.; Nam, J.S. Regulation of wnt signaling activity for growth suppression induced by quercetin in 4t1 murine mammary cancer cells. Int. J. Oncol. 2013, 43, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.T.; Lee, S.H.; Kim, J.I.; Kim, Y.M. Quercetin regulates the sestrin 2-ampk-p38 mapk signaling pathway and induces apoptosis by increasing the generation of intracellular ros in a p53-independent manner. Int. J. Mol. Med. 2014, 33, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Lai, S.L.; Chen, W.S.; Hung, W.Y.; Chow, J.M.; Hsiao, M.; Lee, W.J.; Chien, M.H. Quercetin suppresses the metastatic ability of lung cancer through inhibiting snail-dependent akt activation and snail-independent adam9 expression pathways. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1746–1758. [Google Scholar] [CrossRef]
- Angst, E.; Park, J.L.; Moro, A.; Lu, Q.Y.; Lu, X.; Li, G.; King, J.; Chen, M.; Reber, H.A.; Go, V.L.; et al. The flavonoid quercetin inhibits pancreatic cancer growth in vitro and in vivo. Pancreas 2013, 42, 223–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Khuda-Bukhsh, A.R. Quercetin down-regulates il-6/stat-3 signals to induce mitochondrial-mediated apoptosis in a nonsmall- cell lung-cancer cell line, a549. J. Pharmacopuncture 2015, 18, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Yang, C.; Park, S.; Bazer, F.W.; Song, G. Inhibitory effects of quercetin on progression of human choriocarcinoma cells are mediated through pi3k/akt and mapk signal transduction cascades. J. Cell Physiol. 2017, 232, 1428–1440. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, D.H.; Jeong, J.H.; Guo, Z.S.; Lee, Y.J. Quercetin augments trail-induced apoptotic death: Involvement of the erk signal transduction pathway. Biochem. Pharmacol. 2008, 75, 1946–1958. [Google Scholar] [CrossRef]
- Firdous, A.B.; Sharmila, G.; Balakrishnan, S.; RajaSingh, P.; Suganya, S.; Srinivasan, N.; Arunakaran, J. Quercetin, a natural dietary flavonoid, acts as a chemopreventive agent against prostate cancer in an in vivo model by inhibiting the egfr signaling pathway. Food Funct. 2014, 5, 2632–2645. [Google Scholar] [CrossRef]
- Li, S.; Yuan, S.; Zhao, Q.; Wang, B.; Wang, X.; Li, K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed. Pharmacother. 2018, 100, 441–447. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Reinboth, M.; Wolffram, S.; Abraham, G.; Ungemach, F.R.; Cermak, R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br. J. Nutr. 2010, 104, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.K.; Thanki, K.; Jain, S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: Implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol. Pharm. 2013, 10, 3459–3474. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Ma, Y.; Yu, A.; Cai, F.; Shao, W.; Zhai, G. Formulation optimization and in situ absorption in rat intestinal tract of quercetin-loaded microemulsion. Coll. Surf. B Biointerfaces 2009, 71, 306–314. [Google Scholar] [CrossRef]
- Tan, B.J.; Liu, Y.; Chang, K.L.; Lim, B.K.; Chiu, G.N. Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer. Int. J. Nanomed. 2012, 7, 651–661. [Google Scholar] [Green Version]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef]
- Thakkar, S.; Shah, V.; Misra, M.; Kalia, K. Nanocrystal based drug delivery system: Conventional and current scenario. Recent Pat. Nanotechnol. 2017, 11, 130–145. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Wang, X.; Liu, F.; Xu, Y.; Ma, J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm. 2011, 404, 231–237. [Google Scholar] [CrossRef]
- El-Rahmanand, S.N.A.; Suhailah, S. Quercetin nanoparticles: Preparation and characterization. Indian J. Drugs 2014, 2, 96–103. [Google Scholar]
- Sahoo, N.G.; Kakran, M.; Shaal, L.A.; Li, L.; Müller, R.H.; Pal, M.; Tan, L.P. Preparation and characterization of quercetin nanocrystals. J. Pharm. Sci. 2011, 100, 2379–2390. [Google Scholar] [CrossRef]
- Sun, M.; Gao, Y.; Pei, Y.; Guo, C.; Li, H.; Cao, F.; Yu, A.; Zhai, G. Development of nanosuspension formulation for oral delivery of quercetin. J. Biomed. Nanotechnol. 2010, 6, 325–332. [Google Scholar] [CrossRef]
- Hou, Y.; Cai, K.; Li, J.; Chen, X.; Lai, M.; Hu, Y.; Luo, Z.; Ding, X.; Xu, D. Effects of titanium nanoparticles on adhesion, migration, proliferation, and differentiation of mesenchymal stem cells. Int J. Nanomed. 2013, 8, 3619–3630. [Google Scholar] [Green Version]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- He, C.; Wang, L.; Zhang, J.; Xu, H. Hypoxia-inducible microrna-224 promotes the cell growth, migration and invasion by directly targeting rassf8 in gastric cancer. Mol. Cancer 2017, 16, 35. [Google Scholar] [CrossRef]
- Karanika, S.; Karantanos, T.; Kurosaka, S.; Wang, J.; Hirayama, T.; Yang, G.; Park, S.; Golstov, A.A.; Tanimoto, R.; Li, L.; et al. Glipr1-Δtm synergizes with docetaxel in cell death and suppresses resistance to docetaxel in prostate cancer cells. Mol. Cancer 2015, 14, 122. [Google Scholar] [CrossRef]
- Kumar, R.; Saini, K.S.; Kumar, A.; Kumar, S.; Ramakrishna, E.; Maurya, R.; Konwar, R.; Chattopadhyay, N. Quercetin-6-C-β-D-glucopyranoside, natural analog of quercetin exhibits anti-prostate cancer activity by inhibiting akt-mtor pathway via aryl hydrocarbon receptor. Biochimie 2015, 119, 68–79. [Google Scholar]
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients 2016, 8. [Google Scholar] [CrossRef]
- Lei, C.S.; Hou, Y.C.; Pai, M.H.; Lin, M.T.; Yeh, S.L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: In vitro and in vivo studies. J. Nutr. Biochem. 2018, 51, 105–113. [Google Scholar] [CrossRef]
- Khushnud, T.; Mousa, S.A. Potential role of naturally derived polyphenols and their nanotechnology delivery in cancer. Mol. Biotechnol. 2013, 55, 78–86. [Google Scholar] [CrossRef]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals--special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Nam, J.S.; Doss, G.P.; Lee, S.S.; Chakraborty, C. Nanoparticle based insulin delivery system: The next generation efficient therapy for type 1 diabetes. J. Nanobiotechnol. 2015, 13, 74. [Google Scholar] [CrossRef]
- Sharma, A.R.; Kundu, S.K.; Nam, J.S.; Sharma, G.; Priya Doss, C.G.; Lee, S.S.; Chakraborty, C. Next generation delivery system for proteins and genes of therapeutic purpose: Why and how? Biomed. Res. Int. 2014, 2014, 327950. [Google Scholar] [CrossRef]
- Kakran, M.; Shegokar, R.; Sahoo, N.G.; Gohla, S.; Li, L.; Müller, R.H. Long-term stability of quercetin nanocrystals prepared by different methods. J. Pharm. Pharmacol. 2012, 64, 1394–1402. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Huang, Q. Quercetin nanosuspensions produced by high-pressure homogenization. J. Agric. Food Chem. 2014, 62, 1852–1859. [Google Scholar] [CrossRef]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer efficacy potential of quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef]
- Lakshmi, B.A.; Kim, S. Quercetin mediated gold nanoclusters explored as a dual functional nanomaterial in anticancer and bio-imaging disciplines. Coll. Surf. B Biointerfaces 2019, 178, 230–237. [Google Scholar] [CrossRef]
- Scholz, P.; Keck, C.M. Flavonoid nanocrystals produced by artcrystal®-technology. Int. J. Pharm. 2015, 482, 27–37. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Nanoparticle-cell interactions: Molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.P.; Åberg, C.; Dawson, K.A.; Salvati, A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.R.; Holzapfel, V.; Musyanovych, A.; Nothelfer, K.; Walther, P.; Frank, H.; Landfester, K.; Schrezenmeier, H.; Mailänder, V. Uptake of functionalized, fluorescent-labeled polymeric particles in different cell lines and stem cells. Biomaterials. 2006, 27, 2820–2828. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Y.; Gemeinhart, R.A.; Wu, W.; Li, T. Developing nanocrystals for cancer treatment. Nanomedicine (Lond.) 2015, 10, 2537–2552. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Zou, P.; Wu, M.; Zhang, Z.; Zhang, T. The effects of pharmaceutical excipients on gastrointestinal tract metabolic enzymes and transporters-an update. AAPS J. 2016, 18, 830–843. [Google Scholar] [CrossRef]
- Lee, S.E.; Bairstow, S.F.; Werling, J.O.; Chaubal, M.V.; Lin, L.; Murphy, M.A.; DiOrio, J.P.; Gass, J.; Rabinow, B.; Wang, X.; et al. Paclitaxel nanosuspensions for targeted chemotherapy–nanosuspension preparation, characterization, and use. Pharm. Dev. Technol. 2014, 19, 438–453. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Kang, J.; Niu, M.; Wang, Z.; Wang, H.; Ma, J.; Wang, X. Paclitaxel nanosuspensions coated with p-gp inhibitory surfactants: I. Acute toxicity and pharmacokinetics studies. Coll. Surf. B Biointerfaces 2013, 111, 277–281. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, M.; Morrison, R.A.; Chong, S. Commonly used surfactant, tween 80, improves absorption of p-glycoprotein substrate, digoxin, in rats. Arch. Pharm. Res. 2003, 26, 768–772. [Google Scholar] [CrossRef]
- Nair, H.B.; Sung, B.; Yadav, V.R.; Kannappan, R.; Chaturvedi, M.M.; Aggarwal, B.B. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem. Pharmacol. 2010, 80, 1833–1843. [Google Scholar] [CrossRef]
- Wang, H.; Tao, L.; Qi, K.; Zhang, H.; Feng, D.; Wei, W.; Kong, H.; Chen, T.; Lin, Q. Quercetin reverses tamoxifen resistance in breast cancer cells. J. BUON 2015, 20, 707–713. [Google Scholar]
- Demiroglu-Zergeroglu, A.; Ergene, E.; Ayvali, N.; Kuete, V.; Sivas, H. Quercetin and cisplatin combined treatment altered cell cycle and mitogen activated protein kinase expressions in malignant mesotelioma cells. BMC Complement. Altern. Med. 2016, 16, 281. [Google Scholar] [CrossRef]
- Wang, L.; Du, J.; Zhou, Y.; Wang, Y. Safety of nanosuspensions in drug delivery. Nanomedicine 2017, 13, 455–469. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; de Takats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase i clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar]
Sample Availability: Not available. |






| Number | Milling Speed (rpm) | Milling Time (min) | Size (nm) | PdI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| 1 | 3000 | 4 | 217.28 ± 6.24 | 0.21 ± 0.09 | −28.43 ± 0.85 |
| 2 | 2500 | 2 | 501.51 ± 58.35 | 0.25 ± 0.02 | −21.27 ± 0.59 |
| 3 | 1500 | 4 | 3146.33 ± 105.14 | 0.29 ± 0.06 | −19.43 ± 1.35 |
| Sizes | IC50 (μmol/L) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 200 nm | 47.03 ± 16.64 | 37.88 ± 6.46 | 29.99 ± 6.14 |
| 500 nm | 62.25 ± 15.65 | 48.79 ± 7.95 | 28.59 ± 8.04 |
| 3 μm | 77.06 ± 12.29 | 50.82 ± 11.32 | 28.92 ± 6.76 |
| Concentrations | Adhesion Rate (%) | |||
|---|---|---|---|---|
| 200 nm | 500 nm | 3 μm | Cisplatin | |
| 15 μmol/L | 56.60 ± 16.44 * | 68.53 ± 17.81 * | 74.87 ± 26.43 | / |
| 30 μmol/L | 50.76 ± 12.43 * | 58.79 ± 9.39 * | 63.58 ± 10.11 | / |
| 3 μg/mL | / | / | / | 33.99 ± 7.31 ** |
| Concentrations | Migration Rate (%) | |||
|---|---|---|---|---|
| 200 nm | 500 nm | 3 μm | Cisplatin | |
| 15 μmol/L | 33.00 ± 5.39 **,## | 50.56 ± 2.70 **,## | 68.81 ±4.03 * | / |
| 30 μmol/L | 19.48 ± 3.74 **,## | 42.74 ± 8.08 ** | 50.51 ± 6.75 ** | / |
| 3 μg/mL | / | / | / | 15.32 ± 5.30 ** |
| Concentrations | Invasion Rate (%) | |||
|---|---|---|---|---|
| 200 nm | 500 nm | 3 μm | Cisplatin | |
| 15 μmol/L | 57.17 ± 1.02 **,## | 58.54 ± 9.28 **,# | 81.91 ± 3.08 ** | / |
| 30 μmol/L | 39.97 ± 5.34 **,## | 41.32 ± 4.34 **,## | 70.97 ± 7.29 ** | / |
| 3 μg/mL | / | / | / | 27.09 ± 1.01 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yang, X.; Sun, J.; Yu, F.; Zhang, H.; Gao, J.; Zheng, A. Size-Dependent Biological Effects of Quercetin Nanocrystals. Molecules 2019, 24, 1438. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071438
Liu Q, Yang X, Sun J, Yu F, Zhang H, Gao J, Zheng A. Size-Dependent Biological Effects of Quercetin Nanocrystals. Molecules. 2019; 24(7):1438. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071438
Chicago/Turabian StyleLiu, Qian, Xi Yang, Jianxu Sun, Fanglin Yu, Hui Zhang, Jing Gao, and Aiping Zheng. 2019. "Size-Dependent Biological Effects of Quercetin Nanocrystals" Molecules 24, no. 7: 1438. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071438