Multifloroside Suppressing Proliferation and Colony Formation, Inducing S Cell Cycle Arrest, ROS Production, and Increasing MMP in Human Epidermoid Carcinoma Cell Lines A431
Abstract
:1. Introduction
2. Results
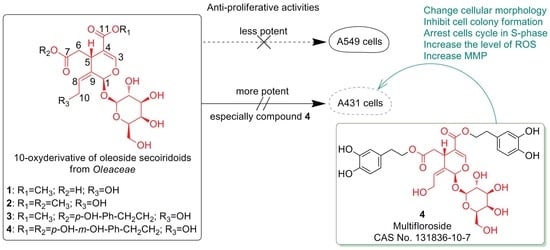
2.1. Anti-Proliferative Activity of 1–4 In Vitro
2.2. The Structure-Activity Relationships (SAR)
2.3. Multifloroside Inhibits Tumor Cell Colony Formation
2.4. Effect of Multifloroside on Cell Apoptosis in A431 Cells
2.5. S-Phase Cell Cycle Arrest Induced by Multifloroside
2.6. Intracellular ROS Production Induced by Multifloroside
2.7. Effect of Multifloroside on the MMP
3. Discussion
4. Methods
4.1. Chemicals and Other Reagents
4.2. Chemicals and Other Reagents
4.3. MTT Assay for In Vitro Anti-Proliferative Activity
4.4. Colony Formation Assay
4.5. Cell Apoptosis Analysis
4.6. Cell Cycle Analysis
4.7. Detection of Intracellular ROS
4.8. Mitochondrial Membrane Potential (MMP) Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Celano, M.; Maggisano, V.; Lepore, S.M.; Russo, D.; Bulotta, S. Secoiridoids of olive and derivatives as potential coadjuvant drugs in cancer: A critical analysis of experimental studies. Pharmacol. Res. 2019, 142, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Wang, F.X.; Jia, K.K.; Kong, L.D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, B.; Satyalakshmi, G. Natural products based anticancer agents. Mini-Rev. Org. Chem. 2012, 9, 169–177. [Google Scholar] [CrossRef]
- Chamberlin, S.R.; Blucher, A.; Wu, G.; Shinto, L.; Choonoo, G.; Kulesz-Martin, M.; McWeeney, S. Natural Product Target Network Reveals Potential for Cancer Combination Therapies. Front. Pharmacol. 2019, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Ling, F.; Wang, Y.; Teng, Y. A natural product atalantraflavone inhibits non-small cell lung cancer progression via destabilizing Twist1. Fitoterapia 2019, 137, 104275. [Google Scholar] [CrossRef]
- Xu, X.; Peng, W.; Liu, C.; Li, S.; Lei, J.; Wang, Z.; Kong, L.; Han, C. Flavone-based natural product agents as new lysine-specific demethylase 1 inhibitors exhibiting cytotoxicity against breast cancer cells in vitro. Bioorg. Med. Chem. 2019, 27, 370–374. [Google Scholar] [CrossRef]
- Huang, P.; Sun, L.Y.; Zhang, Y.Q. A Hopeful Natural Product, Pristimerin, Induces Apoptosis, Cell Cycle Arrest, and Autophagy in Esophageal Cancer Cells. Anal. Cell Pathol. (Amst) 2019, 2019, 6127169. [Google Scholar] [CrossRef] [Green Version]
- Guzman, E.A.; Pitts, T.P.; Diaz, M.C.; Wright, A.E. The marine natural product Scalarin inhibits the receptor for advanced glycation end products (RAGE) and autophagy in the PANC-1 and MIA PaCa-2 pancreatic cancer cell lines. Investig. New Drugs 2019, 37, 262–270. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Yang, X.; Gao, Z.; Song, M.; Han, Y.; Chen, X.; Xiao, H. Synergistic chemopreventive effect of allyl isothiocyanate and sulforaphane on non-small cell lung carcinoma cells. Food Funct. 2019, 10, 893–902. [Google Scholar] [CrossRef]
- Liang, J.W.; Wang, M.Y.; Wang, S.; Li, X.Y.; Meng, F.H. Fragment-Based Structural Optimization of a Natural Product Itampolin A as a p38alpha Inhibitor for Lung Cancer. Mar. Drugs 2019, 17, 53. [Google Scholar] [CrossRef] [Green Version]
- Cowan, J.; Shadab, M.; Nadkarni, D.H.; Kc, K.; Velu, S.E.; Yusuf, N. A Novel Marine Natural Product Derived Pyrroloiminoquinone with Potent Activity against Skin Cancer Cells. Mar. Drugs 2019, 17, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okayama, M.; Kitabatake, S.; Sato, M.; Fujimori, K.; Ichikawa, D.; Matsushita, M.; Suto, Y.; Iwasaki, G.; Yamada, T.; Kiuchi, F.; et al. A novel derivative (GTN024) from a natural product, komaroviquinone, induced the apoptosis of high-risk myeloma cells via reactive oxygen production and ER stress. Biochem. Biophys. Res. Commun. 2018, 505, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Park, M.N.; Song, H.S.; Kim, M.; Lee, M.J.; Cho, W.; Lee, H.J.; Hwang, C.H.; Kim, S.; Hwang, Y.; Kang, B.; et al. Review of Natural Product-Derived Compounds as Potent Antiglioblastoma Drugs. Biomed. Res. Int. 2017, 2017, 8139848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Busselberg, D. Dietary Phytochemicals Targeting Cancer Stem Cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, M.; Machida, K.; Matsuda, N.; Kikuchi, M. A secoiridoid glycoside from Osmanthus asiaticus. Phytochemistry 1993, 34, 1169–1170. [Google Scholar] [CrossRef]
- Sun, J. Studies on the Chemical Constituents of Jasminum lanceolarium and Lobelia sessilifolia, Dissertation Institute of Medicinal Plant Development. Ph.D. Thesis, Peking Union Medical College & Chinese Academy of Medical Sciences, Beijing, China, 2007. [Google Scholar]
- Shen, Y.-C.; Lin, S.-L.; Chein, C.-C. Three secoiridoid glucosides from Jasminum lanceolarium. Phytochemistry 1997, 44, 891–895. [Google Scholar] [CrossRef]
- Shen, Y.C.; Lin, C.Y.; Chen, C.H. Secoiridoid glycosides from Jasminum multiflorum. Phytochemistry 1990, 29, 2905–2912. [Google Scholar] [CrossRef]
- Wang, F.; Han, L.; Song, Y. UPLC/Q-TOF-MS based rapid analysis and identification of chemical composition of Jasminum elongatum (Bergius) Willd. Guangdong Yaoxueyuan Xuebao 2016, 32, 55–60. [Google Scholar]
- Kong, L.Y.; Xue, M.; Zhang, Q.C.; Su, C.F. In vivo and in vitro effects of microRNA-27a on proliferation, migration and invasion of breast cancer cells through targeting of SFRP1 gene via Wnt/beta-catenin signaling pathway. Oncotarget 2017, 8, 15507–15519. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, L.; Xu, H.; Li, X.; Zhao, L.; Wang, W.; Li, B.; Zhang, X. 6,7-Dimorpholinoalkoxy quinazoline derivatives as potent EGFR inhibitors with enhanced antiproliferative activities against tumor cells. Eur. J. Med. Chem. 2018, 147, 77–89. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Sharma, S.; Ojha, R.; Dhar, K.L. Vasicine and structurally related quinazolines. Med. Chem. Res. 2012, 22, 1–15. [Google Scholar] [CrossRef]
- Huang, Y.L.; Oppong, M.B.; Guo, Y.; Wang, L.Z.; Fang, S.M.; Deng, Y.R.; Gao, X.M. The Oleaceae family: A source of secoiridoids with multiple biological activities. Fitoterapia 2019, 136, 104155. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine 1998, 5, 147–163. [Google Scholar] [CrossRef]
- Pérez, J.A.; Hernández, J.M.; Trujillo, J.M.; López, H. Iridoids and secoiridoids from Oleaceae. Stud. Nat. Prod. Chem. 2005, 32, 303–363. [Google Scholar]
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally occurring secoiridoids and bioactivity of naturally occurring iridoids and secoiridoids. A review, part 2. Chem. Pharm. Bull. 2017, 55, 689–728. [Google Scholar] [CrossRef] [Green Version]
- Dinda, B.; Chowdhury, D.R.; Mohanta, B.C. Naturally occurring iridoids, secoiridoids and their bioactivity. An updated review, part 3. Chem. Pharm. Bull. 2009, 57, 765–796. [Google Scholar] [CrossRef] [Green Version]
- Dinda, B.; Debnath, S.; Banik, R. Naturally occurring iridoids and secoiridoids. An updated review, part 4. Chem. Pharm. Bull. 2011, 59, 803–833. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Kim, S.H.; Kim, S.B.; Jo, Y.H.; Kim, E.S.; Hwang, B.Y.; Oh, K.; Lee, M.K. Anti-obesity effect of (8-E)-nuzhenide, a secoiridoid from Ligustrum lucidum, in high-fat diet-induced obese mice. Nat. Prod. Commun. 2014, 9, 1399–1401. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, H.B.; Ahmed, A.A.; Goyal, R.K.; Cheema, S.K. Glycogen phosphorylase-a is a common target for anti-diabetic effect of iridoid and secoiridoid glycosides. J. Pharm. Pharm. Sci. 2013, 16, 530–540. [Google Scholar] [CrossRef] [Green Version]
- Varga, E.; Barabas, C.; Toth, A.; Boldizsar, I.; Noszal, B.; Toth, G. Phenolic composition, antioxidant and antinociceptive activities of Syringa vulgaris L. bark and leaf extracts. Nat. Prod. Res. 2019, 33, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Giardinieri, A.; Schicchi, R.; Geraci, A.; Rosselli, S.; Maggi, F.; Fiorini, D.; Ricciutelli, M.; Loizzo, M.R.; Bruno, M.; Pacetti, D. Fixed oil from seeds of narrow-leaved ash (F. angustifolia subsp. angustifolia): Chemical profile, antioxidant and antiproliferative activities. Food Res. Int. 2019, 119, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Jin, M.; Li, R.; Diao, S.; Sun, J.; Ma, Y.J.; Zhou, W.; Li, G. Isolation of a new natural kingiside aglucone derivative and other anti-inflammatory constituents from Syringa reticulate. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Pinto-Bustillos, M.A.; Vasquez-Ponce, P.; Prodanov, M.; Martinez-Rodriguez, A.J. Olive mill wastewater as a potential source of antibacterial and anti-inflammatory compounds against the food-borne pathogen Campylobacter. Innovative Food Sci. Emerging Technol. 2019, 51, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Suh, W.S.; Kwon, O.K.; Lee, T.H.; Subedi, L.; Kim, S.Y.; Lee, K.R. Secoiridoid glycosides from the twigs of Ligustrum obtusifolium possess anti-inflammatory and neuroprotective effects. Chem. Pharm. Bull. 2018, 66, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, Q.-M.T.; Lee, H.-S.; Nguyen, V.T.; Woo, M.H.; Kim, J.A.; Min, B.S. Chemical constituents from the fruits of Ligustrum japonicum and their inhibitory effects on T cell activation. Phytochemistry 2017, 141, 147–155. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Kulsi, G.; Chakraborty, A.; Dinda, S. Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: A review. Eur. J. Med. Chem. 2019, 169, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Suh, W.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Secoiridoid Glucosides from the Twigs of Syringa oblata var. dilatata and Their Neuroprotective and Cytotoxic Activities. Chem. Pharm. Bull. 2017, 65, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Hao, E.-W.; Zhang, M.; Pan, X.-L.; Qin, J.-F.; Xie, J.-L.; Deng, J.-G.; Wei, W.; Hao, E.-W.; Zhang, M.; et al. Chemical constituents from Jasminum pentaneurum Hand.-Mazz and their cytotoxicity against human cancer cell lines. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Essafi Rhouma, H.; Trabelsi, N.; Chimento, A.; Benincasa, C.; Tamaalli, A.; Perri, E.; Zarrouk, M.; Pezzi, V. Olea europaea L. Flowers as a new promising anticancer natural product: phenolic composition, antiproliferative activity and apoptosis induction. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Fabiani, R. Anti-cancer properties of olive oil secoiridoid phenols: A systematic review of in vivo studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M.; Mojzis, J.; Blahutova, D.; Qaradakhi, T.; Zulli, A.; et al. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Hobbs, M.; Meyn, R.E. Clonogenic cell survival assay. Methods Mol. Med. 2005, 110, 21–28. [Google Scholar] [PubMed]
- Nieddu, V.; Pinna, G.; Marchesi, I.; Sanna, L.; Asproni, B.; Pinna, G.A.; Bagella, L.; Murineddu, G. Synthesis and Antineoplastic Evaluation of Novel Unsymmetrical 1,3,4-Oxadiazoles. J. Med. Chem. 2016, 59, 10451–10469. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, Y.; Xu, Z.; Wang, J.; Chen, B.; Jin, H. Potential antitumor effects of panaxatriol against DU-15 human prostate cancer cells is mediated via mitochondrial mediated apoptosis, inhibition of cell migration and sub-G1 cell cycle arrest. J. BUON 2018, 23, 200–204. [Google Scholar]
- Niu, H.; Li, X.; Yang, A.; Jin, Z.; Wang, X.; Wang, Q.; Yu, C.; Wei, Z.; Dou, C. Cycloartenol exerts anti-proliferative effects on Glioma U87 cells via induction of cell cycle arrest and p38 MAPK-mediated apoptosis. J. BUON 2018, 23, 1840–1845. [Google Scholar]
- Xu, S.; Yao, H.; Luo, S.; Zhang, Y.-K.; Yang, D.-H.; Li, D.; Wang, G.; Hu, M.; Qiu, Y.; Wu, X.; et al. A Novel Potent Anticancer Compound Optimized from a Natural Oridonin Scaffold Induces Apoptosis and Cell Cycle Arrest through the Mitochondrial Pathway. J. Med. Chem. 2017, 60, 1449–1468. [Google Scholar] [CrossRef]
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatrilova, R.; Kruzliak, P.; Stefanicka, P.; Busselberg, D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. 2018, 101, 458–477. [Google Scholar] [CrossRef]
- Lou, C.; Xu, X.; Chen, Y.; Zhao, H.; Alisol, A. Suppresses Proliferation, Migration, and Invasion in Human Breast Cancer MDA-MB-231 Cells. Molecules 2019, 24, 3651. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Zhang, T.; Yuan, H.; Li, D.; Lou, H.; Fan, P. Mitochondria-Targeted Lupane Triterpenoid Derivatives and Their Selective Apoptosis-Inducing Anticancer Mechanisms. J. Med. Chem. 2017, 60, 6353–6363. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Q.; Li, X.; Zhu, J.; Wang, W.; Li, B.; Zhao, L.; Xia, H. Enrichment of novel quinazoline derivatives with high antitumor activity in mitochondria tracked by its self-fluorescence. Eur. J. Med. Chem. 2019, 178, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Kroon, P.A.; Shao, H.; Needs, P.W.; Yang, X. Differential Effects of Quercetin and Two of Its Derivatives, Isorhamnetin and Isorhamnetin-3-glucuronide, in Inhibiting the Proliferation of Human Breast-Cancer MCF-7 Cells. J. Agric. Food. Chem. 2018, 66, 7181–7189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Liu, J.; Chen, L.; Zhao, L.; Li, B.; Wang, W. Synthesis and in vitro biological evaluation of novel quinazoline derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.; Liu, J.; Wang, W.; Li, X.; Zhao, L.; Wang, W.; Li, B. Novel 4-arylaminoquinazoline derivatives with (E)-propen-1-yl moiety as potent EGFR inhibitors with enhanced antiproliferative activities against tumor cells. Eur. J. Med. Chem. 2017, 138, 689–697. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, Y.; Feng, Z.; Zhang, Y.; Gong, Y.; Song, H.; Ding, X.; Yan, Y. Multifloroside Suppressing Proliferation and Colony Formation, Inducing S Cell Cycle Arrest, ROS Production, and Increasing MMP in Human Epidermoid Carcinoma Cell Lines A431. Molecules 2020, 25, 7. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25010007
Zhang X, Li Y, Feng Z, Zhang Y, Gong Y, Song H, Ding X, Yan Y. Multifloroside Suppressing Proliferation and Colony Formation, Inducing S Cell Cycle Arrest, ROS Production, and Increasing MMP in Human Epidermoid Carcinoma Cell Lines A431. Molecules. 2020; 25(1):7. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25010007
Chicago/Turabian StyleZhang, Xin, Yamei Li, Zhengping Feng, Yaling Zhang, Ye Gong, Huanhuan Song, Xiaoli Ding, and Yaping Yan. 2020. "Multifloroside Suppressing Proliferation and Colony Formation, Inducing S Cell Cycle Arrest, ROS Production, and Increasing MMP in Human Epidermoid Carcinoma Cell Lines A431" Molecules 25, no. 1: 7. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25010007