Resveratrol: A Fair Race Towards Replacing Sulfites in Wines
Abstract
:1. Introduction
2. Wine Sulfites and Human Health
3. The Use of Sulfur Dioxide (SO2) in Winemaking
4. Disadvantages of the Use of Sulfur Dioxide in Winemaking
5. SO2 Replacement in Wines
6. Resveratrol Content in Wine
7. Positive Effects of Resveratrol Intake on Consumer Health
8. Antioxidant Activity of Resveratrol in Wines
9. Antimicrobial Activity of Resveratrol in Wine
10. The Solubility of Resveratrol in Wine as a Limiting Factor for Its Industrial Application
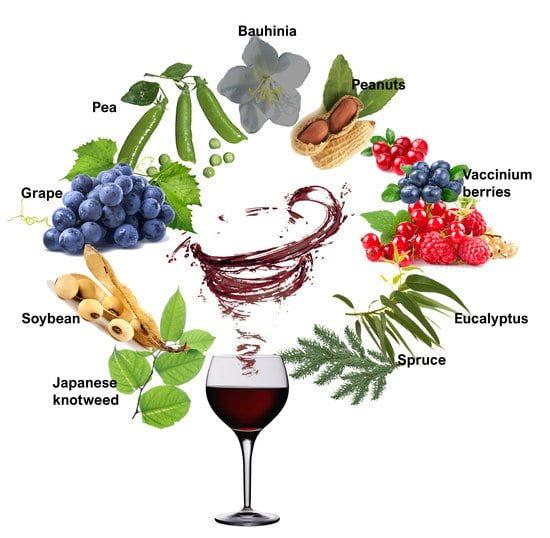
11. Potential Sources of Resveratrol for Commercial Production
12. Innovative Microbial Biosynthesis of Resveratrol through Metabolic Engineering
13. Alternative Biotechnological Production of Resveratrol through Plant Cell Suspensions
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rossi, A.; Fusco, F. Wine Index of Salubrity and Health (WISH): An evidence-based instrument to evaluate the impact of good wine on well-being. Int. J. Wine Res. 2019, 11, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Pappalardo, G.; Di Vita, G.; Zanchini, R.; La Via, G.; D’Amico, M. Do consumers care about antioxidants in wine? The role of naturally resveratrol-enhanced wines in potential health-conscious drinkers’ preferences. Br. Food J. 2019. [Google Scholar] [CrossRef]
- Moreno, A.; Castro, M.; Falqué, E. Evolution of trans-and cis-resveratrol content in red grapes (Vitis vinifera L. cv Mencía, Albarello and Merenzao) during ripening. Eur. Food Res. Technol. 2008, 227, 667–674. [Google Scholar] [CrossRef]
- Vitaglione, P.; Sforza, S.; Galaverna, G.; Ghidini, C.; Caporaso, N.; Vescovi, P.P.; Fogliano, V.; Marchelli, R. Bioavailability of trans-resveratrol from red wine in humans. Mol. Nutr. Food Res. 2005, 49, 495–504. [Google Scholar] [CrossRef]
- Vivancos, M.; Moreno, J.J. Effect of resveratrol, tyrosol and β-sitosterol on oxidised low-density lipoprotein-stimulated oxidative stress, arachidonic acid release and prostaglandin E 2 synthesis by RAW 264.7 macrophages. Br. J. Nutr. 2008, 99, 1199–1207. [Google Scholar] [CrossRef] [Green Version]
- Dudley, J.I.; Lekli, I.; Mukherjee, S.; Das, M.; Bertelli, A.A.A.; Das, D.K. Does White Wine Qualify for French Paradox? Comparison of the Cardioprotective Effects of Red and White Wines and Their Constituents: Resveratrol, Tyrosol, and Hydroxytyrosol. J. Agric. Food Chem. 2008, 56, 9362–9373. [Google Scholar] [CrossRef]
- Kaeberlein, M.; McDonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S.; et al. Substrate-specific Activation of Sirtuins by Resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.H.; Kumar, R.; Ahmad, N. Cancer chemoprevention by resveratrol: In vitro and in vivo studies and the underlying mechanisms. Int. J. Oncol. 2003, 23, 17–28. [Google Scholar] [CrossRef]
- Trantas, E.; Panopoulos, N.; Ververidis, F. Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab. Eng. 2009, 11, 355–366. [Google Scholar] [CrossRef]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part II: Reconstruction of multienzyme pathways in plants and microbes. Biotechnol. J. 2007, 2, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- Ferrières, J. The French paradox: Lessons for other countries. Heart 2004, 90, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottart, C.H.; Nivet-Antoine, V.; Beaudeux, J.L. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol. Nutr. Food Res. 2014, 58, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Novelle, M.G.; Wahl, D.; Dieguez, C.; Bernier, M.; de Cabo, R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Khakimov, B.; Engelsen, S.B. Resveratrol in the foodomics era: 1: 25,000. Ann. N. Y. Acad. Sci. 2017, 1403, 48–58. [Google Scholar] [CrossRef]
- Giacosa, S.; Río Segade, S.; Cagnasso, E.; Caudana, A.; Rolle, L.; Gerbi, V. Chapter 21—SO2 in Wines: Rational use and possible alternatives. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 309–321. [Google Scholar]
- Peroni, D.G.; Boner, A.L. Sulfite sensitivity. Clin. Exp. Allergy 1995, 25, 680–681. [Google Scholar] [CrossRef]
- Galati, A.; Schifani, G.; Crescimanno, M.; Migliore, G. “Natural wine” consumers and interest in label information: An analysis of willingness to pay in a new Italian wine market segment. J. Clean. Prod. 2019, 227, 405–413. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of enology. In The Microbiology of Wine and Vinifications, 2nd ed.; Wiley: Chichester, UK, 2006; Volume 1, pp. 193–220. [Google Scholar]
- Scampicchio, M.; Lawrence, N.S.; Arecchi, A.; Mannino, S. Determination of Sulfite in Wine by Linear Sweep Voltammetry. Electroanalysis 2008, 20, 444–447. [Google Scholar] [CrossRef]
- Taylor, S.L.; Higley, N.A.; Bush, R.K. Sulfites in Foods: Uses, Analytical Methods, Residues, Fate, Exposure Assessment, Metabolism, Toxicity, and Hypersensitivity. In Advances in Food Research; Chichester, C.O., Mrak, E.M., Schweigert, B.S., Eds.; Academic Press: Cambridge, MA, USA, 1986; Volume 30, pp. 1–76. [Google Scholar]
- Granchi, L.; Budroni, M.; Rauhut, D.; Zara, G. Wine Yeasts and Consumer Health. In Yeasts in the Production of Wine; Romano, P., Ciani, M., Fleet, G.H., Eds.; Springer: New York, NY, USA, 2019; pp. 343–373. [Google Scholar]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- López-Seijas, J.; García-Fraga, B.; da Silva, A.F.; Sieiro, C. Wine Lactic Acid Bacteria with Antimicrobial Activity as Potential Biocontrol Agents against Fusarium oxysporum f. sp. lycopersici. Agronomy 2019, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Costanigro, M.; Appleby, C.; Menke, S.D. The wine headache: Consumer perceptions of sulfites and willingness to pay for non-sulfited wines. Food Qual. Prefer. 2014, 31, 81–89. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the evaluation of allergenic foods and food ingredients for labelling purposes. EFSA J. 2014, 12. [Google Scholar] [CrossRef]
- Benito, S. The management of compounds that influence human health in modern winemaking from an HACCP point of view. Fermentation 2019, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Cravero, M.C. Organic and biodynamic wines quality and characteristics: A review. Food Chem. 2019, 295, 334–340. [Google Scholar] [CrossRef]
- Bazzani, C.; Capitello, R.; Ricci, E.C.; Scarpa, R.; Begalli, D. Nutritional knowledge and health consciousness: Do they affect consumer wine choices? Evidence from a survey in Italy. Nutrients 2019, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, M.; Di Vita, G.; Monaco, L. Exploring environmental consciousness and consumer preferences for organic wines without sulfites. J. Clean. Prod. 2016, 120, 64–71. [Google Scholar] [CrossRef]
- Lester, M.R. Sulfite sensitivity: Significance in human health. J. Am. Coll. Nutr. 1995, 14, 229–232. [Google Scholar] [CrossRef]
- Bacchetti, T.; Annibaldi, A.; Comitini, F.; Ciani, M.; Damiani, E.; Norici, A.; Tiano, L.; Truzzi, C.; Olivotto, I. Alternative Ingredients for Feed and Food. In The First Outstanding 50 Years of “Università Politecnica Delle Marche”: Research Achievements in Life Sciences; Longhi, S., Monteriù, A., Freddi, A., Aquilanti, L., Ceravolo, M.G., Carnevali, O., Giordano, M., Moroncini, G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 529–545. [Google Scholar]
- Staub, C.; Michel, F.; Bucher, T.; Siegrist, M. How do you perceive this wine? Comparing naturalness perceptions of Swiss and Australian consumers. Food Qual. Prefer. 2020, 79. [Google Scholar] [CrossRef]
- Garaguso, I.; Nardini, M. Polyphenols content, phenolics profile and antioxidant activity of organic red wines produced without sulfur dioxide/sulfites addition in comparison to conventional red wines. Food Chem. 2015, 179, 336–342. [Google Scholar] [CrossRef]
- Bleotu, C.; Mambet, C.; Matei, L.; Dragu, L.D. 14—Improving Wine Quality and Safety Through Nanotechnology Applications. In Nanoengineering in the Beverage Industry; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 437–458. [Google Scholar]
- Roberts, A.; McWeeny, D. The uses of sulphur dioxide in the food industry: A review. Int. J. Food Sci. Technol. 1972, 7, 221–238. [Google Scholar] [CrossRef]
- Morgan, S.C.; Tantikachornkiat, M.; Scholl, C.M.; Benson, N.L.; Cliff, M.A.; Durall, D.M. The effect of sulfur dioxide addition at crush on the fungal and bacterial communities and the sensory attributes of Pinot gris wines. Int. J. Food Microbiol. 2019, 290, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Martin-Alvarez, P.J.; Reglero, G.; Herraiz, M.; Cabezudo, M.D. Differences between wines fermented with and without sulphur dioxide using various selected yeasts. J. Sci. Food Agric. 1989, 49, 249–258. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Sulfur Dioxide. In Production Wine Analysis; Springer: Berlin/Heidelberg, Germany, 1990; pp. 185–206. [Google Scholar]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Eschenbruch, R. Sulfite and sulfide formation during winemaking—A review. Am. J. Enol. Viticult. 1974, 25, 157–161. [Google Scholar]
- Jackson, R.S. Wine Science: Principles and Applications (Food Science and Technology), 4th ed.; Academic Press; Elsevier Inc.: New York, NY, USA, 2014. [Google Scholar]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Campaniello, D.; Sinigaglia, M. Chapter 10—Wine Spoiling Phenomena. In The Microbiological Quality of Food; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing: Duxford, UK, 2017; pp. 237–255. [Google Scholar]
- Jackson, R.S. Chemical Constituents of Grapes and Wine. In Wine Science: Principle, Practice, Perception, 2nd ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 232–280. [Google Scholar]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative methods to SO2 for microbiological stabilization of wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Gallego, R.; Puxeu, M.; Martín, L.; Nart, E.; Hidalgo, C.; Andorrà, I. Chapter 9- Microbiological, physical, and chemical procedures to elaborate high-quality SO2-free wines. In Grapes and Wines: Advances in Production, Processing, Analysis and Valorization, 1st ed.; IntechOpen: London, UK, 2018; pp. 171–185. [Google Scholar]
- Sovak, M. Grape Extract, Resveratrol, and Its Analogs: A Review. J. Med. Food 2001, 4, 93–105. [Google Scholar] [CrossRef]
- Fernández-Mar, M.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Melzoch, K.; Hanzlíková, I.; Filip, V.; Buckiová, D.; Šmidrkal, J. Resveratrol in parts of vine and wine originating from Bohemian and Moravian vineyard regions. Agric. Conspec. Sci. 2001, 66, 53–57. [Google Scholar]
- Mohidul, H.; Hanhong, B. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Shao, Y.; Marriott, P.; Hügel, H. Solid-phase microextraction—On-fibre derivatization with comprehensive two dimensional gas chromatography analysis oftrans-resveratrol in wine. Chromatographia 2003, 57, 349–353. [Google Scholar] [CrossRef]
- Gu, X.; Creasy, L.; Kester, A.; Zeece, M. Capillary electrophoretic determination of resveratrol in wines. J. Agric. Food Chem. 1999, 47, 3223–3227. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.M.; Ng, E.; Karumanchiri, A.; Diamandis, E.P.; Soleas, G.J. Resveratrol glucosides are important components of commercial wines. Am. J. Enol. Viticult. 1996, 47, 415–420. [Google Scholar]
- Souto, A.A.; Carneiro, M.C.; Seferin, M.; Senna, M.J.; Conz, A.; Gobbi, K. Determination of trans-resveratrol concentrations in Brazilian red wines by HPLC. Food Compos. Anal. 2001, 14, 441–445. [Google Scholar] [CrossRef]
- Kallithraka, S.; Arvanitoyannis, I.; El-Zajouli, A.; Kefalas, P. The application of an improved method for trans-resveratrol to determine the origin of Greek red wines. Food Chem. 2001, 75, 355–363. [Google Scholar] [CrossRef]
- Dourtoglou, V.G.; Makris, D.P.; Bois-Dournas, F.; Zonas, C. Trans-resveratrol Concentration in Wines Produced in Greece. Food Compos. Anal. 1999, 12, 227–233. [Google Scholar] [CrossRef]
- Gerogiannaki-Christopoulou, M.; Athanasopoulos, P.; Kyriakidis, N.; Gerogiannaki, I.A.; Spanos, M. trans-Resveratrol in wines from the major Greek red and white grape varieties. Food Control 2006, 17, 700–706. [Google Scholar] [CrossRef]
- Mark, L.; Pour Nikfardjam, M.; Avar, P.; Ohmacht, R. A validated HPLC method for the quantitative analysis of trans-Resveratrol and trans-Piceid in Hungarian wines. J. Chromatogr. Sci. 2005, 43, 445–449. [Google Scholar] [CrossRef]
- Abril, M.; Negueruela, A.; Pérez, C.; Juan, T.; Estopañán, G. Preliminary study of resveratrol content in Aragón red and rosé wines. Food Chem. 2005, 92, 729–736. [Google Scholar] [CrossRef]
- Lamuela-Raventos, R.M.; Romero-Perez, A.I.; Waterhouse, A.L.; De La Torre-Boronat, M.C. Direct HPLC analysis of cis-and trans-resveratrol and piceid isomers in Spanish red Vitis vinifera wines. J. Agric. Food Chem. 1995, 43, 281–283. [Google Scholar] [CrossRef]
- Martínez-Ortega, M.V.; Carcía-Parrilla, M.C.; Troncoso, A.M. Resveratrol content in wines and musts from the south of Spain. Food Nahr. 2000, 44, 253–256. [Google Scholar] [CrossRef]
- Gürbüz, O.; Göçmen, D.; Dagdelen, F.; Gürsoy, M.; Aydin, S.; Şahin, İ.; Büyükuysal, L.; Usta, M. Determination of flavan-3-ols and trans-resveratrol in grapes and wine using HPLC with fluorescence detection. Food Chem. 2007, 100, 518–525. [Google Scholar] [CrossRef]
- Roldán, A.; Palacios, V.; Caro, I.; Pérez, L. Resveratrol Content of Palomino fino Grapes: Influence of Vintage and Fungal Infection. J. Agric. Food Chem. 2003, 51, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Cvejić, J.; Atanacković, M. Chapter 60—Effect of Wine Production Techniques on Wine Resveratrol and Total Phenolics. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 501–508. [Google Scholar]
- Clare, S.S.; Skurray, G.; Shalliker, R.A. Effect of pomace-contacting method on the concentration of cis-and trans-resveratrol and resveratrol glucoside isomers in wine. Am. J. Enol. Viticult. 2004, 55, 401–406. [Google Scholar]
- Vrhovsek, U.; Wendelin, S.; Eder, R. Effects of various vinification techniques on the concentration of cis-and trans-resveratrol and resveratrol glucoside isomers in wine. Am. J. Enol. Viticult. 1997, 48, 214–219. [Google Scholar]
- Clare, S.; Skurray, G.; Shalliker, A. Effect of yeast strain selection on the concentration of cis- and trans-resveratrol and resveratrol glucoside isomers in wine. Aust. J. Grape Wine Res. 2005, 11, 9–14. [Google Scholar] [CrossRef]
- Pastor, R.F.; Gargantini, M.R.; Murgo, M.; Prieto, S.; Manzano, H.; Aruani, C.; Quini, C.I.; Covas, M.-I.; Iermoli, R.H. Enrichment of resveratrol in wine through a new vinification procedure. J. Life Sci. 2015, 9, 327–333. [Google Scholar]
- Gaudette, N.J.; Pickering, G.J. Sensory and chemical characteristics of trans-resveratrol-fortified wine. Aust. J. Grape Wine Res. 2011, 17, 249–257. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef] [Green Version]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular Protective Effects of Resveratrol. Cardiovasc. Drug Rev. 2004, 22, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, G.; Gurusamy, N.; Das, D.K. Resveratrol in cardiovascular health and disease. Ann. N. Y. Acad. Sci. 2011, 1215, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Arslan, A.K.K.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Kuršvietienė, L.; Stanevičienė, I.; Mongirdienė, A.; Bernatonienė, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef]
- Guerrero, R.F.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Wine, Resveratrol and Health: A Review. Nat. Prod. Commun. 2009, 4, 635–658. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Artero, A.; Artero, A.; Tarín, J.J.; Cano, A. The impact of moderate wine consumption on health. Maturitas 2015, 80, 3–13. [Google Scholar] [CrossRef]
- Barreiro-Hurlé, J.; Colombo, S.; Cantos-Villar, E. Is there a market for functional wines? Consumer preferences and willingness to pay for resveratrol-enriched red wine. Food Qual. Prefer. 2008, 19, 360–371. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Saliba, A.J.; Prenzler, P.D. Should Red Wine Be Considered a Functional Food? Compr. Rev. Food. Sci. Food Saf. 2010, 9, 530–551. [Google Scholar] [CrossRef]
- Laurie, V.F.; Clark, A.C. Wine Oxidation; Woodhead Publishing Ltd.: Cambridge, UK, 2010; pp. 445–475. [Google Scholar]
- López-Vélez, M.; Martínez-Martínez, F.; Valle-Ribes, C.D. The Study of Phenolic Compounds as Natural Antioxidants in Wine. Crit. Rev. Food Sci. Nutr. 2003, 43, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Troncoso, A.M.; García-Parrilla, M.C. Comparison of antioxidant activity of wine phenolic compounds and metabolites in vitro. Anal. Chim. Acta 2005, 538, 391–398. [Google Scholar] [CrossRef]
- Murcia, M.A.; Martinez-Tome, M. Antioxidant Activity of Resveratrol Compared with Common Food Additives. J. Food Prot. 2001, 64, 379–384. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Pormohammad, A.; Chirani, A.S.; Pouriran, R.; Erfanimanesh, S.; Hashemi, A. Comprehensive review on the antimicrobial potency of the plant polyphenol Resveratrol. Biomed. Pharmacother. 2017, 95, 1588–1595. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bartolomé, B. Comparative study of the inhibitory effects of wine polyphenols on the growth of enological lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 426–431. [Google Scholar] [CrossRef]
- Pastorkova, E.; Zakova, T.; Landa, P.; Novakova, J.; Vadlejch, J.; Kokoska, L. Growth inhibitory effect of grape phenolics against wine spoilage yeasts and acetic acid bacteria. Int. J. Food Microbiol. 2013, 161, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Sabel, A.; Bredefeld, S.; Schlander, M.; Claus, H. Wine phenolic compounds: Antimicrobial properties against yeasts, lactic acid and acetic acid bacteria. Beverages 2017, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Filip, V.; Plocková, M.; Šmidrkal, J.; Špičková, Z.; Melzoch, K.; Schmidt, Š. Resveratrol and its antioxidant and antimicrobial effectiveness. Food Chem. 2003, 83, 585–593. [Google Scholar] [CrossRef]
- Dietrich, H.; Pour Nikfardjam, M.S. Influence of Phenolic Compounds and Tannins on Wine-Related Microorganisms. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 421–454. [Google Scholar]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol encapsulation: Designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Milner, J. Functional foods and health promotion. J. Nutr. 1999, 129, 1395–1397. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F. Functional foods: Their role in health promotion and disease prevention. J. Food Sci. 2004, 69, 146–149. [Google Scholar] [CrossRef]
- Martínez, A.V.; García, J.I.; Mayoral, J.A. An expedient synthesis of resveratrol through a highly recoverable palladium catalyst. Tetrahedron 2017, 73, 5581–5584. [Google Scholar] [CrossRef] [Green Version]
- El-Deeb, I.Y.; Funakoshi, T.; Shimomoto, Y.; Matsubara, R.; Hayashi, M. Dehydrogenative Formation of Resorcinol Derivatives Using Pd/C–Ethylene Catalytic System. J. Org. Chem. 2017, 82, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Duenas, M.; Hernandez, T.; Estrella, I. Assessment of in vitro antioxidant capacity of the seed coat and the cotyledon of legumes in relation to their phenolic contents. Food Chem. 2006, 98, 95–103. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tuck, T.; Ji, X.; Zhou, X.; Kelly, G.; Cuerrier, A.; Zhang, J. Quality assessment of Japanese knotweed (Fallopia japonica) grown on Prince Edward Island as a source of resveratrol. J. Agric. Food Chem. 2013, 61, 6383–6392. [Google Scholar] [CrossRef]
- Rolfs, C.H.; Kindl, H. Stilbene Synthase and Chalcone Synthase: Two Different Constitutive Enzymes in Cultured Cells of Picea excelsa. Plant Physiol. 1984, 75, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P.; et al. Plants of the genus Vitis: Phenolic compounds, anticancer properties and clinical relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Rayne, S.; Karacabey, E.; Mazza, G. Grape cane waste as a source of trans-resveratrol and trans-viniferin: High-value phytochemicals with medicinal and anti-phytopathogenic applications. Ind. Crops Prod. 2008, 27, 335–340. [Google Scholar] [CrossRef]
- Mei, Y.-Z.; Liu, R.-X.; Wang, D.-P.; Wang, X.; Dai, C.-C. Biocatalysis and biotransformation of resveratrol in microorganisms. Biotechnol. Lett. 2015, 37, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Trantas, E.A.; Koffas, M.; Xu, P.; Ververidis, F. When plants produce not enough or at all: Metabolic engineering of flavonoids in microbial hosts. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Otero, J.M.; Nielsen, J. Industrial systems biology. Biotechnol. Bioeng. 2010, 105, 439–460. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E. Resveratrol production at large scale using plant cell suspensions. Eng. Life Sci. 2014, 14, 622–632. [Google Scholar] [CrossRef]
- Delaunois, B.; Cordelier, S.; Conreux, A.; Clement, C.; Jeandet, P. Molecular engineering of resveratrol in plants. Plant Biotechnol. J. 2009, 7, 2–12. [Google Scholar] [CrossRef]
- Schulz, W.; Eiben, H.-G.; Hahlbrock, K. Expression in Escherichia coli of catalytically active phenylalanine ammonia-lyase from parsley. FEBS Lett. 1989, 258, 335–338. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.V.; Armstrong, G.O.; van der Merwe, M.J.; Lambrechts, M.G.; Vivier, M.A.; Pretorius, I.S. Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res. 2003, 4, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.G.; Fowler, Z.L.; Hueller, T.; Schaffer, S.; Koffas, M.A. High-yield resveratrol production in engineered Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 3451–3460. [Google Scholar] [CrossRef] [Green Version]
- Sydor, T.; Schaffer, S.; Boles, E. Considerable increase in resveratrol production by recombinant industrial yeast strains with use of rich medium. Appl. Environ. Microbiol. 2010, 76, 3361–3363. [Google Scholar] [CrossRef] [Green Version]
- Bhan, N.; Xu, P.; Khalidi, O.; Koffas, M.A. Redirecting carbon flux into malonyl-CoA to improve resveratrol titers: Proof of concept for genetic interventions predicted by OptForce computational framework. Chem. Eng. Sci. 2013, 103, 109–114. [Google Scholar] [CrossRef]
- Beekwilder, J.; Wolswinkel, R.; Jonker, H.; Hall, R.; de Vos, C.H.; Bovy, A. Production of resveratrol in recombinant microorganisms. Appl. Environ. Microbiol. 2006, 72, 5670–5672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, K.T.; Lee, P.C.; Schmidt-Dannert, C. Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli. BMC Biotechnol. 2006, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, O.; Wu, C.Z.; Kang, S.Y.; Ahn, J.S.; Uhm, T.B.; Hong, Y.S. Biosynthesis of plant-specific phenylpropanoids by construction of an artificial biosynthetic pathway in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2011, 38, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-Y.; Jung, S.-M.; Kim, M.-D.; Han, N.S.; Seo, J.-H. Production of resveratrol from tyrosine in metabolically engineered Saccharomyces cerevisiae. Enzyme Microb. Technol. 2012, 51, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Halls, C.; Zhang, J.; Matsuno, M.; Zhang, Y.; Yu, O. Stepwise increase of resveratrol biosynthesis in yeast Saccharomyces cerevisiae by metabolic engineering. Metab. Eng. 2011, 13, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Zaragoza, J.M.; Hernandez-Chavez, G.; Moreno-Avitia, F.; Ramírez-Iñiguez, R.; Martínez, A.; Bolívar, F.; Gosset, G. Engineering of a microbial coculture of Escherichia coli strains for the biosynthesis of resveratrol. Microb. Cell Fact. 2016, 15. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Kildegaard, K.R.; Chen, Y.; Rodriguez, A.; Borodina, I.; Nielsen, J. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab. Eng. 2015, 32, 1–11. [Google Scholar] [CrossRef]
- Wang, M.; Jin, Y.; Ho, C.-T. Evaluation of resveratrol derivatives as potential antioxidants and identification of a reaction product of resveratrol and 2, 2-diphenyl-1-picryhydrazyl radical. J. Agric. Food Chem. 1999, 47, 3974–3977. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Lee, J.K.; Choi, O.; Kim, C.Y.; Jang, J.-H.; Hwang, B.Y.; Hong, Y.-S. Biosynthesis of methylated resveratrol analogs through the construction of an artificial biosynthetic pathway in E. coli. BMC Biotechnol. 2014, 14. [Google Scholar] [CrossRef] [Green Version]
- Choi, O.; Lee, J.K.; Kang, S.-Y.; Pandey, R.P.; Sohng, J.-K.; Ahn, J.S.; Hong, Y.-S. Construction of artificial biosynthetic pathways for resveratrol glucoside derivatives. J. Microbiol. Biotechnol. 2014, 24, 614–618. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yu, O. Synthetic scaffolds increased resveratrol biosynthesis in engineered yeast cells. J. Biotechnol. 2012, 157, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Conrado, R.J.; Wu, G.C.; Boock, J.T.; Xu, H.; Chen, S.Y.; Lebar, T.; Turnšek, J.; Tomšič, N.; Avbelj, M.; Gaber, R. DNA-guided assembly of biosynthetic pathways promotes improved catalytic efficiency. Nucleic Acids Res. 2011, 40, 1879–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuyama, Y.; Funa, N.; Miyahisa, I.; Horinouchi, S. Synthesis of unnatural flavonoids and stilbenes by exploiting the plant biosynthetic pathway in Escherichia coli. Chem. Biol. 2007, 14, 613–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinowski, J.; Bathe, B.; Bartels, D.; Bischoff, N.; Bott, M.; Burkovski, A.; Dusch, N.; Eggeling, L.; Eikmanns, B.J.; Gaigalat, L. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J. Biotechnol. 2003, 104, 5–25. [Google Scholar] [CrossRef]
- Braga, A.; Oliveira, J.; Silva, R.; Ferreira, P.; Rocha, I.; Kallscheuer, N.; Marienhagen, J.; Faria, N. Impact of the cultivation strategy on resveratrol production from glucose in engineered Corynebacterium glutamicum. J. Biotechnol. 2018, 265, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Kallscheuer, N.; Vogt, M.; Stenzel, A.; Gätgens, J.; Bott, M.; Marienhagen, J. Construction of a Corynebacterium glutamicum platform strain for the production of stilbenes and (2S)-flavanones. Metab. Eng. 2016, 38, 47–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.Z.; Li, J.; Pan, X.; Cahoon, R.E.; Jaworski, J.G.; Wang, X.; Jez, J.M.; Chen, F.; Yu, O. Using unnatural protein fusions to engineer resveratrol biosynthesis in yeast and mammalian cells. J. Am. Chem. Soc. 2006, 128, 13030–13031. [Google Scholar] [CrossRef]
- Park, S.R.; Yoon, J.A.; Paik, J.H.; Park, J.W.; Jung, W.S.; Ban, Y.H.; Kim, E.J.; Yoo, Y.J.; Han, A.R.; Yoon, Y.J. Engineering of plant-specific phenylpropanoids biosynthesis in Streptomyces venezuelae. J. Biotechnol. 2009, 141, 181–188. [Google Scholar] [CrossRef]
- Maharjan, S.; Park, J.W.; Yoon, Y.J.; Lee, H.C.; Sohng, J.K. Metabolic engineering of Streptomyces venezuelae for malonyl-CoA biosynthesis to enhance heterologous production of polyketides. Biotechnol. Lett. 2010, 32, 277–282. [Google Scholar] [CrossRef]
- Oksman-Caldentey, K.-M.; Inzé, D. Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant. Sci. 2004, 9, 433–440. [Google Scholar] [CrossRef]
- Donnez, D.; Jeandet, P.; Clement, C.; Courot, E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Belchí-Navarro, S.; Pedreño, M.; Almagro, L. Critical parameters on which the production of trans-resveratrol in Vitis vinifera cv Monastrell cell cultures depends. Plant Cell Tissue Organ Cult. 2019, 138, 395–398. [Google Scholar] [CrossRef]
- Lambert, C.; Lemaire, J.; Auger, H.; Guilleret, A.; Reynaud, R.; Clément, C.; Courot, E.; Taidi, B. Optimize, modulate, and scale-up resveratrol and resveratrol dimers bioproduction in Vitis labrusca L. cell suspension from flasks to 20 L bioreactor. Plants 2019, 8, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Summeren-Wesenhagen, P.V.; Marienhagen, J. Metabolic engineering of Escherichia coli for the synthesis of the plant polyphenol pinosylvin. Appl. Environ. Microbiol. 2015, 81, 840–849. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |


| Region | Limit | Regulatory |
|---|---|---|
| Australia | 250 mg/L for wines containing <35 g/L sugars 300 mg/L for wines containing ≥35 g/L sugars | Australia New Zealand Food Standards Code–Standard 4.5.1: Clause 5(5)(a) |
| Canada | 70 mg/L for all wines (free SO2) 350 mg/L for all wines (total SO2) | Can. Food and Drug Regulations (C.R.C., c. 870), B.02.100 |
| European Union | 150 mg/L for red wines (containing ≤5 g/L of sugar content) 200 mg/L for white and rosé wines (containing ≤5 g/L of sugar content) 200 mg/L for red wines (containing >5 g/L of sugar content) 250 mg/L for white and rosé wines (containing >5 g/L of sugar content) 300 mg/L exceptionally in certain wines, described in Reg. (EU) 2019/934 | Regulation (EU) 2019/934 |
| India | 450 mg/L for all wines | The Prevention of Food Adulteration Act & Rules |
| Japan | 350 mg/Kg for all wines | Japan′s Specifications and Standards for Food Additives |
| New Zealand | 250 mg/Kg for wines containing <35 g/L sugars 400 mg/Kg for wines containing ≥35 g/L sugars | Australia New Zealand Food Standards Code–Standard 4.5.1: Clause 5(5)(a) |
| South Africa | 160 mg/L for white wines (containing <5 g/L of sugars) 150 mg/L for red wines (containing <5 g/L of sugars) 200 mg/L for all wines (containing ≥5 g/L of sugars) 300 mg/L exceptionally for noble late harvest wine and wine from naturally dried grapes | Liquor Products Act 60 Regulation 32, Table 8, Note 2 |
| United States of America | 350 mg/L for all wines (total SO2) | Code of Federal Regulations, 27 CFR § 4.22 |
| World–International Organisation of Vine and Wine (OIV) | 150 mg/L for red wines (containing ≤4 g/L of reducing substances) 200 mg/L for white and rosé wines (containing ≤4 g/L of reducing substances) 300 mg/L for red, rosé and white wines (containing >4 g/L of reducing substances) 400 mg/L exceptionally in certain sweet white wines | International Code of Oenological Practices (Issue 2019) |
| Country | Wine Color | trans-Resveratrol (mg/L) | Sample Number | Reference | |
|---|---|---|---|---|---|
| Range | Mean | ||||
| Australia | Red | 0.100–0.950 | 0.440 | 5 | [54] |
| Red | 1.461–1.548 | 1.504 | 2 | [55] | |
| Red | 2.371 | 35 | [56] | ||
| Brazil | Red | 0.820–5.750 | 2.570 | 36 | [57] |
| California | Red | 0.226–2.319 | 0.864 | 8 | [55] |
| Red | 1.685 | 72 | [56] | ||
| France | Red | 1.735–2.901 | 1.966 | 4 | [55] |
| Red | 2.085 | 48 | [56] | ||
| Greece | Red | 0.550–2.534 | 1.105 | 29 | [58] |
| Red | 0.325–1.569 | 0.873 | 15 | [59] | |
| White | 0.026–0.142 | 0.043 | 15 | [59] | |
| Red | 0.352–1.991 | 0.895 | 13 | [60] | |
| White | 0.005–0.571 | 0.229 | 18 | [60] | |
| Hungary | Red | 0.100–14.300 | 2.380 | 68 | [61] |
| White | 0.200–0.780 | 0.563 | 3 | [61] | |
| Italy | Red | 0.657–1.155 | 0.984 | 3 | [55] |
| Spain | Red | 0.320–4.440 | 1.471 | 74 | [62] |
| Rosé | 0.120–2.800 | 0.669 | 24 | [62] | |
| Red | 0.600–8.000 | 2.485 | 18 | [63] | |
| Red | <0.012–0.472 | 0.179 | 14 | [64] | |
| White | <0.012–0.062 | 0.024 | 8 | [64] | |
| Turkey | Red | 0.176–4.403 | 1.203 | 7 | [65] |
| White | 0.116–1.243 | 0.891 | 4 | [65] | |
| Species | MIC (mg/L) | Reference | |
|---|---|---|---|
| Yeasts | Debaryomyces hansenii | 250 | [96] |
| Dekkera bruxellensis | 256 | [95] | |
| Hanseniaspora uvarum | 256 | [95] | |
| Saccharomyces bayanus | 250 | [96] | |
| S. cerevisiae | 250 | [96] | |
| 256 | [95] | ||
| S. cerevisiae × S. kudriavzevii × S. bayanus | 250 | [96] | |
| Wickerhamomyces anomalus | 250–500 | [96] | |
| Zygosaccharomyces bailii | 256 | [95] | |
| Z. rouxii | 512 | [95] | |
| Acetic Acid Bacteria | Acetobacter acetii | 250 | [96] |
| 256 | [95] | ||
| A. oeni | 256 | [95] | |
| A. pasteurianus | 256 | [95] | |
| Gluconobacter cerinus | >1000 | [96] | |
| Lactic Acid Bacteria | Lactobacillus hilgardii | 250 855 | [96] [94] |
| L. plantarum | 250 | [96] | |
| Oenococcus oeni | 250 | [96] | |
| 307–698 | [94] | ||
| Pediococcus parvulus | 250 | [96] | |
| P. pentosaceus | 715 | [94] |
| End Product | Precursor Molecule (Alternative Source) | Number of Genes | Target Genes and Sources | Strategy | Host Organism | Production Level (mg/L) | Reference |
|---|---|---|---|---|---|---|---|
| Resveratrol | NA | 3 | Rhodobacter sphaeroides (TAL), Arabidopsis thaliana (4CL), Vitis vinifera (RS) | MOC GO FUS | Human HEK293 cells | 0.0283 | [131] |
| Resveratrol | Phenylalanine | 5 | Populus trichocarpa x P.deltoides (PAL, CPR), Glycine max (C4H, 4CL), Vitis vinifera (RS) | MOC GO | Saccharomyces cerevisiae | 0.29 | [9] |
| Resveratrol | Galactose | 3 | Rhodobacter sphaeroides (TAL), Arabidopsis thaliana (4CL), Vitis vinifera (RS) | MOC GO OPT FUS | S. cerevisiae | 1.06 | [130] |
| Resveratrol | Tyrosine | 3 | Saccharothrix espanaensis (TAL), Streptomyces coelicolor (4CL), Arachis hypogaea (RS) | MOC GO OPT | Escherichia coli | 1.4 | [128] |
| Resveratrol | Galactose (Tyrosine) | 4 | Rhodosporidium toruloides (PAL), A. thaliana (C4H), A. thaliana (4CL), A. hypogaea (RS), S. cerevisiae (ACC1) | MOC GO IPCO | S. cerevisiae | 4.3 (5.8) | [129] |
| Resveratrol | Glycerol | 5 | E. coli (AroG, tktA), Rhodothorula glutinis (TAL), S. coelicolor (4CL), (STS), ΔpheA | COC GO OPM KOG | E. coli | 22.6 | [132] |
| Resveratrol | Glucose (Ethanol) | 6 | Herpetosiphon aurantiacus (TAL), Arabidopsis thaliana (4CL), Vitis vinifera (RS), S. cerevisiae (ARO4), S. cerevisiae (ARO7), S. cerevisiae (ACC1) | MOC GOCI BIOR OPM IPCO | S. cerevisiae | 415.65 (531.41) | [133] |
| Resveratrol | Glucose | 4 | E. coli (AroH) Flavobacterium johnsoniae (TAL) A. hypogaea (STS), Petroselinum crispum (4CL), ΔqsuB | MOC KOG OPT GOCI GO | Corynebacterium glutamicum | 12 | [134,135] |
| Pinosylvin | Glucose (phenylalanine) | 3 | P. crispum (PAL), S. coelicolor (4CL), Pinus strobus (STS) | MOC OPT GO CER | E. coli | 70 (91) | [136] |
| Methylated Resveratrol derivatives | Glucose | 5 | S. espanaensis (TAL), Streptomyces coelicolor (4CL), Stilbene synthase (STS), Sorghum bicolor (OMT), | MOC GO OPT | E. coli | NE | [137] |
| Mix of 3 glucosylated Resveratrol derivatives | Glucose | 4 | S. espanaensis (TAL), S. coelicolor (4CL), Arachis hypogaea (STS), Bacillus licheniformis (YjiC) | MOC GO OPT | E. coli | 11.7 | [138] |
| Resveratrol | NA | NA | NA | Cell suspension | V. vinifera cv. Monastrell | 2140 | [139] |
| Resveratrol | NA | NA | NA | Cell suspension in flasks (Bioreactor) | V. vinifera cv. Labrusca | 6141 (4230) | [140] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontaxakis, E.; Trantas, E.; Ververidis, F. Resveratrol: A Fair Race Towards Replacing Sulfites in Wines. Molecules 2020, 25, 2378. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102378
Kontaxakis E, Trantas E, Ververidis F. Resveratrol: A Fair Race Towards Replacing Sulfites in Wines. Molecules. 2020; 25(10):2378. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102378
Chicago/Turabian StyleKontaxakis, Emmanouil, Emmanouil Trantas, and Filippos Ververidis. 2020. "Resveratrol: A Fair Race Towards Replacing Sulfites in Wines" Molecules 25, no. 10: 2378. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102378